After wreaking havoc on a pandemic level for more than three years, COVID-19 has entered its first endemic season. The glorious days of COVID products are gone as companies cut costs, record revenue declines and refocus their attention on other areas. But having worked on a COVID product or not, people in the biopharma industry powered through a health crisis when the world expected the most from them. And that’s worth remembering.
A few months ago, Fierce Pharma asked employees working in the biopharma industry to use photographs to tell their stories during the pandemic. Now, we’re showcasing those memories here.
Many stories are directly about developing or producing a COVID drug, vaccine or diagnostic; some recap how companies supported their patients or the general public. A few others talk about finding new ways to work during the pandemic. Some photos are very personal, while others might deliver a high-level review of a business.
We weren’t able to include all photos we had received, and we couldn’t cover every aspect of biopharma’s experience during the pandemic. But we hope this article could serve as biopharma’s COVID yearbook, one that could jog each person’s unique memory of the challenging times.

Barbara Rodriguez Spong still remembers the Sunday call she received from Pfizer managers following a separate call with the company’s CEO, Albert Bourla, Ph.D. During the discussions, the Pfizer executives discussed the potential repurposing of existing drugs for COVID. As a director of drug product manufacturing at Pfizer’s facility in Groton, Connecticut, Rodriguez Spong and her team produced drugs for scientists to test in the lab and for patients who were enrolled in clinical trials. Early in the pandemic, her parents’ infections created a very personal sense of urgency to help find a treatment. Pfizer responded to the situation by implementing new digital tools and workflows to protect workers and speed production operations. Under these circumstances, Paxlovid was developed in “light speed—in timelines we have never seen before with other solid dosage forms,” she said.

Just two days after the World Health Organization declared COVID-19 a pandemic, Regeneron’s protein expression sciences team successfully isolated viable B cells from the blood samples of early coronavirus survivors. After an overnight endeavor, the scientists extracted two unique anti-coronavirus antibodies from two patients. One of the antibodies later became a component used in REGEN-COV—known as Ronapreve outside the U.S.—which in November 2020 became the first antibody combo to receive an emergency use authorization from the FDA for COVID.

There’s designing a COVID vaccine, and then there’s actually making the doses. James Dyer II, a senior manager in manufacturing sciences at AstraZeneca’s lab in Gaithersburg, Maryland, started working on the company’s COVID shot in May 2020 and played a critical role in the clinical development of the vaccine, Vaxzevria. Dyer's job was to produce a large stock of virus materials in bioreactors. As part of this effort, Dyer infected host cells and let the virus replicate before purifying the material to make the master virus stock, which would then be used to make the vaccine. Before infecting the cells, Dyer took samples from the bioreactor to count the host cells, because the process requires a very specific ratio of virus to host cells. During the pandemic, AZ and its production partners supplied more than 3 billion doses of Vaxzevria around the globe.

Before rapid COVID tests became widely available, doctors largely relied on medical imaging tools such as CT scans to assess cases. But as infections rose quickly, imaging experts became overwhelmed. In response to a February 2020 request from a team in Wuhan, China, GE HealthCare engineers—many working from home in China and the U.S.—came up with a new “CT in a Box” solution. The entire process, from conceptualization to installation of the first product in the city, took less than 20 days. The portable equipment offered medical imaging with safe distancing measures and minimized contact between clinicians and test subjects. More than 100 “boxes” such the one shown in the photo were then installed in several countries.

COVID-related lockdowns caused many disruptions to pharma’s supply chain. It was against this backdrop that an urgent request arrived at Novartis from a hospital in Berlin. Two critical blood cancer patients needed to be treated with the Swiss pharma’s personalized cell therapy Kymriah. Given the urgency and anticipated delay in the usual delivery channel, Joerg Trappe, Ph.D., a Novartis cell therapy operations manager, rented a car and personally transported the complicated Kymriah equipment nearly 560 miles from Basel, Switzerland, to Berlin. Upon arrival and after gaining multiple approvals, Trappe worked alongside the treatment teams to guide them through cell collection and eventually infusing Kymriah back to the patients.

Many people joined the pharma industry during the pandemic. Frank Duff, now senior vice president of virology at Gilead Sciences, was one of them. As a physician and then infectious disease researcher, Duff joined Gilead’s fight against COVID in April 2020. One of Duff’s key jobs was ensuring that, beyond randomized clinical trials, real-world evidence and insights were being captured as the pandemic evolved. “Preparing for the next health threat and supporting access to the latest innovative therapies for all people remains critical work and keeps me motivated in this unexpected chapter of my life,” he said.

Even before a phase 3 readout, Merck & Co. started ramping up production of the drug that would eventually become the company’s oral COVID antiviral Lagevrio. A plant in Wilson, North Carolina, was key to Merck’s manufacturing network. There, Merck employees such as Otis Farr worked long hours to prepare the medicine for shipments. By the end of 2021, when Lagevrio won an FDA emergency use authorization to treat people with mild to moderate COVID, Merck had already produced 10 million courses of the therapy, representing the fastest manufacturing scale-up in the company’s history. The drug has treated more than 4.9 million people worldwide.

For many parents, working from home while taking care of young children was a challenging pandemic experience. But for Ganesh Ramachandran, Ph.D., a chief scientific manager working on peptides R&D at Biocon, it was a reminder of what’s important. His home workspace was brightened up by his 10-year-old son’s colorful artworks, with an extra touch of charm from his daughter’s doll house and toys. “Having my family’s presence and support motivated me to stay productive and put my best into my work,” Ramachandran said. “It also reminded me of the importance of gratitude and appreciating the simple pleasures of life. Being in a safe and secure environment surrounded by people who love and care for you creates a sense of blessing and contentment.”

Italy was among those most severely hit by the first wave of COVID in early 2020. At this time, Michele Mangolini, a customer application specialist of Roche Diagnostics in Italy, offered technical assistance to local labs, including the San Raffaele Hospital in Milan. Amid an influx of tests, Mangolini was entrusted to help labs manage incoming COVID samples to make sure results were obtained as quickly as possible for doctors and patients. Early detection of COVID played a key role in preventing the spread of the virus during the early days of the pandemic. Roche received FDA emergency use authorization for its cobas SARS-CoV-2 nucleic acid test in March 2020 and then for its Elecsys coronavirus antibody test the following May.

Scientists at AI drug discovery company Insilico Medicine were among those who rushed to identify new potential treatments for COVID during the pandemic. As the company’s AI worked around the clock to design new compounds, founder and CEO Alex Zhavoronkov and colleagues slept in Insilico’s lab in Shanghai. Eventually, Insilico chose a novel 3CL protease inhibitor, which was optimized from compounds designed by the company’s AI platform, Chemistry42. In February 2023, the drug, coded ISM3312, received clearance from Chinese authorities to begin a first-in-human clinical trial.

Mass immunization with COVID-19 vaccines was key to the battle against the virus. While AbbVie doesn’t have a COVID vaccine, its employees contributed their expertise to the task of immunization. In Illinois, where the company is headquartered, about 600 AbbVie employees helped administer about 12,000 doses of COVID vaccines at a drive-thru clinic built on the Lake County fairgrounds. The company also worked with the local government to launch a temporary vaccination clinic in north Chicago that vaccinated almost 6,000 seniors.
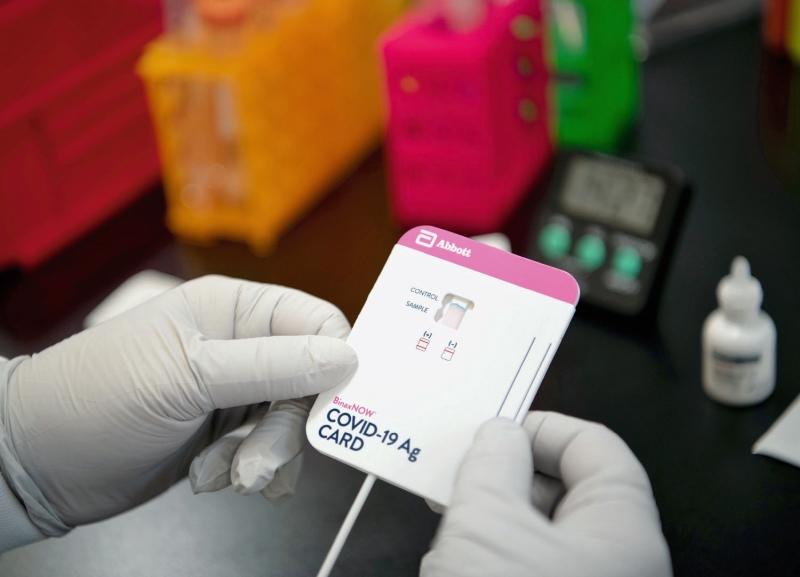
During the pandemic, Abbott developed 12 different COVID tests, including its BinaxNOW rapid test. The BinaxNOW professional test rolled out in August 2020, and the FDA in March 2021 issued an emergency use authorization for its over-the-counter version for individuals with or without symptoms. To meet global demand for COVID diagnostics, Abbott built two new manufacturing facilities in the U.S. in a matter of months. Such installments would typically take years to complete. All told, Abbott delivered nearly 3 billion COVID tests throughout the pandemic.

The pandemic led to a surge in demand for Merck KGaA’s life sciences business, MilliporeSigma, which provides equipment and related services to biopharma companies. Filtration devices and membranes manufactured in the company’s Jaffrey, New Hampshire, facility were used by MilliporeSigma’s customers to produce therapies and vaccines. The company’s Danvers, Massachusetts, site (shown in photo) took on new responsibilities to make single-use consumables used in bioreactors. The German company was also responsible for producing lipids, which are used to deliver Pfizer and BioNTech’s COVID vaccine, Comirnaty.

As a contract research organization, Parexel helps companies run clinical trials. In the pandemic’s early days, COVID significantly disrupted how clinical trials were normally conducted, causing delays or even failures in studies of non-COVID drugs. At this time, Parexel’s early-phase clinical unit team in Baltimore navigated many challenges. As some trials inevitably hit pause, Parexel helped drug developers get compliant with regulators while preparing other sponsors to adopt to decentralized methodologies.
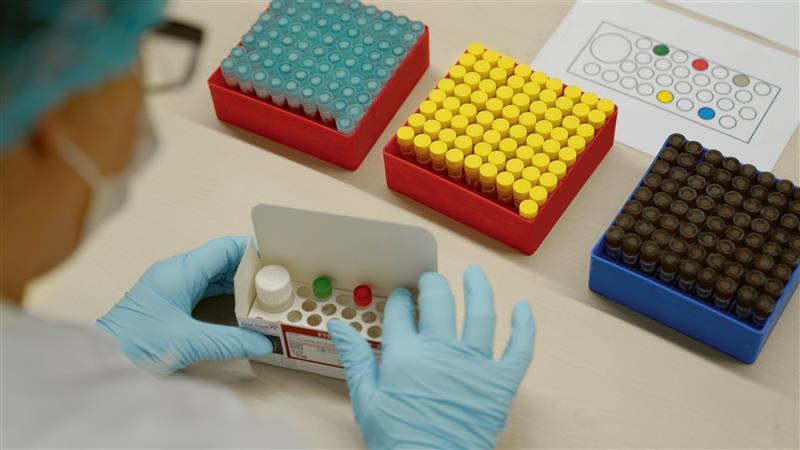
In response to the pandemic, Siemens Healthineers developed a portfolio of diagnostics, including a total antibody test to detect both immunoglobulin M and immunoglobulin G antibodies to SARS-CoV-2 in the blood and a real-time PCR assay for molecular laboratories. Additional blood gas and imaging solutions also helped clinicians better care for COVID patients.

With travel restrictions in place, on-site manufacturing inspections became an almost impossible task for the FDA. As a contract manufacturer, Samsung Biologics developed Live Virtual Tour, a platform that allowed the FDA and its clients to remotely observe and assess its facilities and documents. Following its launch in 2020, the platform enabled one of the FDA’s earliest virtual inspections for emergency use authorization of COVID treatments and six pre-approval inspections in just seven days under the European Medicine Agency’s distant assessment pathway.