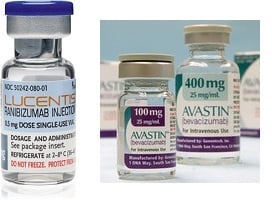
 Italian finance police searched the offices of the Italian Medicines Agency (Aifa), looking for evidence in a criminal probe of potential fraud and market manipulation by the Swiss drugmakers Novartis ($NVS) and Roche ($RHHBY). Officials have been investigating allegations that the two companies colluded to boost sales of Lucentis, their eye drug, by sidelining off-label use of the drug's close cousin, Avastin.
Italian finance police searched the offices of the Italian Medicines Agency (Aifa), looking for evidence in a criminal probe of potential fraud and market manipulation by the Swiss drugmakers Novartis ($NVS) and Roche ($RHHBY). Officials have been investigating allegations that the two companies colluded to boost sales of Lucentis, their eye drug, by sidelining off-label use of the drug's close cousin, Avastin.
Italian authorities say that Roche and Novartis operated as a "cartel," persuading health officials to nix Avastin as an eye treatment in favor of the much more expensive Lucentis. French and EU officials are each conducting their own investigations into the matter. Both drugs are made by Roche, and Lucentis is marketed by Novartis outside the U.S.
Italy has already levied $251 million in fines on the two companies. Now, the country is looking for an additional 1.2 billion euros in damages, or about $1.5 billion. The Aifa raid stems from allegations that the two companies worked together to put Lucentis on the Italian formulary to treat vision-robbing conditions--and to exclude Avastin for the same uses. Aifa itself may also be a target in the probe; according to the Italian news agency ANSA, a consumer association in the country said in March that it would urge state auditors to investigate Aifa's potential role in the alleged cartel.
Using Lucentis rather than Avastin cost Italy's national health service more than €45 million in 2012--and could eventually cost €600 million ($815 million) a year, investigators have said. Earlier this week, a Health Affairs study concluded that the U.S. could save $3 billion by substituting Avastin for Lucentis, which was specifically developed for use in the eye, partly to minimize side effects.
The Lucentis vs. Avastin question has a long history. Roche's Genentech unit allegedly threw up barriers to ophthalmic use of Avastin in the U.S. by cutting off distribution to ophthalmologists. The company says Lucentis is safer than Avastin for eye injections, because Avastin has to be repackaged in syringes for that use, and that repackaging can cause infections. Several clusters of serious eye infections have been traced to repackaged Avastin.
Meanwhile, the National Institutes of Health funded a head-to-head study of the two drugs to see whether Avastin really is an effective substitute, and the study authors concluded that it is more-or-less equally effective, albeit with some differences in side effects. But Lucentis is FDA-approved for eye use and Avastin is not, and Medicare payments tend to encourage eye doctors to use Lucentis for wet AMD and other eye conditions.
Across the Atlantic, cost-effectiveness watchdogs in the U.K. approved Lucentis for use by the National Health Service, though Novartis ended up negotiating a discount to make sure Lucentis would be adopted by physicians. Switzerland demanded--and got--a Lucentis discount as well, after complaining of the big price difference between it and Avastin.
- see the ANSA news
Special Reports: The 15 best-selling drugs of 2012 - Avastin | The top 10 pharma companies by 2013 revenue - Roche - Novartis