Respiratory devicemaker PARI picked up an approval from the FDA for its eRapid nebulizer system, which is the first electronic one to deliver Genentech's cystic fibrosis treatment Pulmozyme.
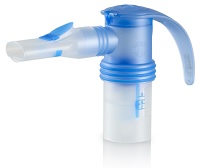 |
| A nebulizer from PARI featuring the eRapid platform--Courtesy of PARI |
The eRapid system is designed to deliver particles of a consistent size targeted toward the lungs very quickly, bringing treatment time for Pulmozyme down to two to three minutes, according to a release. Average treatment for the drug without the device hovered around 6 to 8 minutes, the company said.
"We have been pleased with eRapid's fast treatment times in the lab and are excited that patients now have access to a much faster Pulmozyme therapy," partnering director Lisa Cambridge said in a statement. "As the first electronic nebulizer to deliver Pulmozyme, eRapid is a true breakthrough for cystic fibrosis patients who take the therapy daily, often for years."
The delivery system, part of a slate of nebulizers offered by PARI, has been distributed in Europe for years with a high approval rate among patients, the company reports.
Roche ($RHHBY), Genentech's parent company, put up $8.3 billion last year to bolster its lung drug business with InterMune's Esbriet, which joined Pulmozyme and Xolair in Genentech's lineup. PARI's recent approval gives that business a boost.
"After the successful results of a Phase IV study, we are confident that physicians will see that both pediatric and adult patients favor eRapid based on reduced treatment times, quiet operation, and its small, portable size," Cambridge said. " We were also happy to see that patients were more satisfied with treatment and eRapid had a positive influence on adherence--good for their overall cystic fibrosis management."
- here's the release