 |
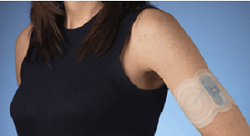
| NuPathe's migraine patch Zecuity--Courtesy of NuPathe |
With the headache of a previous FDA rejection behind it, NuPathe ($PATH) finally gained the agency's approval for its migraine patch, Zecuity, a battery-powered delivery system designed to get a tried-and-true drug into the bloodstream.
The patch, which uses electricity to exchange ions with the skin to deliver the migraine drug sumatriptan, had some flaws in its last design, which gave some patients minor burns due to exposed electrodes. The approved model has a pad detection system that prevents such a reaction, NuPathe CEO Armando Anido told FierceDrugDelivery.
"There were 7 patients out of 10,000 who had small burns from the patch ... and the FDA told us to go ahead and fix it," Anido said of the August 2011 rejection. "After the redesign, they complimented us on it."
The idea behind the patch, the first of its kind to deliver sumatriptan, is that migraine patients, who may show cases of nausea because of the condition, can avoid swallowing an oral med and instead absorb the drug through the process of iontophoresis.
The company is looking for a commercial partner as it goes ahead with manufacturing, Anido said, and plans are to make the patch available in the fourth quarter of this year.
- here's the release
- see Bloomberg's article