 |
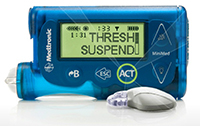
| Medtronic's MiniMed with Threshold Suspend technology--Courtesy of Medtronic |
In the scurry to get an artificial pancreas to the market, Medtronic ($MDT) had something of a leg up--the Minnesota-based med tech giant had two iterations of the insulin delivery device in the works by the middle of this year. And the company took a giant leap forward Friday as the FDA approved one of those versions, its MiniMed 530G device with Threshold Suspend technology.
While the auto-shutoff feature doesn't quite make this MiniMed the fully automated, closed-loop artificial pancreas the industry has been chasing for years, its approval does represent the beginning of next-generation insulin delivery, as well as a major success in the field for Medtronic.
The device is now the closest thing to an artificial pancreas with FDA approval. The MiniMed 530G is less advanced than a true closed-loop system in that it relies on a continuous glucose monitor only to shut the pump down when blood sugar reaches a preset level. This is a safeguard against insulin overdose but not representative of the complete automation associated with an artificial pancreas.
Medtronic's next-gen MiniMed device with a closed-loop system is undergoing an 85-patient study for overnight use and stands in direct competition with similar technologies from Johnson & Johnson ($JNJ), Becton Dickinson ($BD) and Tandem Diabetes Care.
Medtronic's device makes use of the company's Threshold Suspend technology to ensure accurate and timely shutoff. This is a product of the its Enlite sensor, which the company touts as its most sensitive glucose sensor with 31% more accuracy from the previous generation, according to a release. When blood sugar levels trigger an abnormal response from the sensor, an alarm sounds first to alert the user, but if there is no response--if the patient is sleeping or unconscious--then the pump shuts down automatically. The Enlite sensor is designed to detect up to 93% of hypoglycemia episodes, the company says, and can be worn for 6 days at a time.
Medtronic plans to ramp up production immediately and launch the device in the next few weeks. For now, the MiniMed 530G is relegated to the 16-and-up age group, but the company is conducting a post-approval study in children as young as 2.
"We're excited to bring yet another important 'first' to the United States," Katie Szyman, president of Medtronic's diabetes arm, said in a statement. "The MiniMed 530G with Enlite can help people gain better control of their diabetes versus multiple daily injections. We are committed to advancing closed-loop algorithms, continuous glucose monitoring and insulin delivery technologies to bring new artificial pancreas systems to market."
A representative from Medtronic could not be reached by press time.
- here's the release
- and here's FierceMedicalDevices' take
Special Report: The race for the artificial pancreas
Related Articles:
Study: Artificial pancreas with inhaled insulin improves glucose control
Roundup: Insulin delivery devices at ADA
Artificial pancreas draft guidance could hurt Medtronic, others