The success--or failure--of still-unproven biotech BioDeliverySciences ($BDSI) will depend in great part on its dissolvable buccal film for opioid dependence, Bunavail. The candidate is barreling toward its June 6 PDUFA date.
CEO Mark Sirgo told FierceDrugDelivery he is confident that the product can grab a large chunk of the $1.7 billion market from competitor Suboxone if the product is approved.
The active ingredient in both drugs is buprenorphine--a member of a class of medications used to treat opioid addiction--but Bunavail is delivered via the inner cheek whereas Reckitt Benckiser's Suboxone is taken under the tongue. Sirgo said Bunavail is more convenient because the patient does not notice the medication's presence in the cheek, while those using Suboxone are not supposed to swallow for 10 minutes.
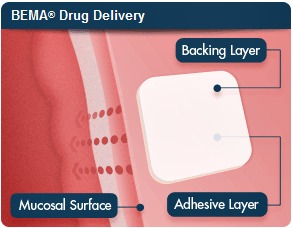
BioDeliverySciences' BEMA technology delivers buprenorphine to the bloodstream via a polymer film that attaches to the mucous membrane and dissolves within 15 to 30 minutes, according to the company's website.
Sirgo said that because Bunavail has superior absorbability to the bloodstream, it contains less buprenorphine, resulting in fewer side effects. He pointed to study results showing that Bunavail patients had a lower rate of constipation than those who were on Suboxone.
The CEO is "cautiously optimistic" of approval. Contract research organization Quintiles ($Q) will provide the sales force to support the product launch, assuming Bunavail is approved.
The BEMA delivery technology is used in BioDeliverySciences' commercially available opioid agonist Onsolis for the management of cancer pain.