 |
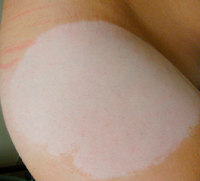
| Chemical leukoderma--Courtesy FDA |
The FDA warned that a transdermal patch used to treat ADHD in children and adolescents can lead to chemical leukoderma, or permanent skin discoloration, as a result of the skin's repeated exposure to specific chemical compounds.
The new warning on the labeling pokes at the Daytrana patch's main differentiating feature: drug delivery. Noven Pharmaceuticals touts the long-lasting product as an alternative to pills, saying it overcomes issues like pill-swallowing. Because the patch delivers the stimulant methylphenidate directly to the bloodstream, absorption isn't affected by a child's diet or eating routine. Daytrana can be worn for up to 9 hours, eliminating the need for multiple oral doses throughout the day.
But it turns out that the patch can cause new areas of lighter skin that are up to 8 inches in diameter, especially under the patch, which is worn in the hip area, the FDA says. The agency's adverse event reporting system received 51 reports between April 2006 and December 2014, and other instances likely went unreported. In 7 of the reported cases, the depigmentation occurred in other areas of the body in addition to the application site.
Among patients with the side effect, alternative treatment options should be considered, the FDA said in a safety communication notice, adding that the condition may cause emotional distress. The agency also recommended placing the patch on different parts of the hip and switching sides daily so that no one area of the skin is exposed to the transdermal system on a regular basis.
"The time to onset of leukoderma after starting Daytrana ranged from 2 months to 4 years. All of the patients described a decrease in or loss of skin color," the FDA said in the notice. "In most cases, the loss of skin color was limited to the areas around where the patch was rotated. However, a small number of patients also reported skin color changes on parts of the body where the patch was never applied. In all cases, the decreased skin color was permanent."
Daytrana was prescribed to 109,000 patients in 2014, down from 136,500 patients in 2010, according to the FDA. Other side effects include increased blood pressure and heart rate, nausea, decreased appetite and weight, aggressive behavior, anger, and irritability.
- read the FDA's safety alert
- here's a more detailed explanation from the FDA