Online pharmacies are a huge headache for the FDA. They often sell illegal, misbranded, even counterfeit drugs delivered in small volume usually sent through the postal service. That makes them terribly hard to intercept. On top of that, under rules designed with large manufacturers and exporters in mind, the FDA has to return the products they seize if the company appeals and asks for them back.
But that is about to change according to Regulatory Focus, which points to a new rule the FDA intends to implement. The agency is claiming the authority to get rid of any drugs that are worth less than $2,500 and which, after an appeal, have been refused entry. The originators would be notified of FDA's intent and could still appeal, but if they lose that, then the products would be destroyed.
The proposal speaks to the difficulty that the FDA and other federal authorities face in even dealing with the issue. It points out that there is about 1.2 million pieces of international mail coming into the U.S. on average each day. Between 20 million and 100 million each year contain illegal drugs and the FDA seized 14,000 after checking over three times that many. But under rules that allow drug manufacturers to get back any product refused at the borders, the FDA has been shipping some of these back, only to have them delivered again to the U.S., sometimes in the same package bearing the FDA "refusal" identifier, Regulatory Focus said.
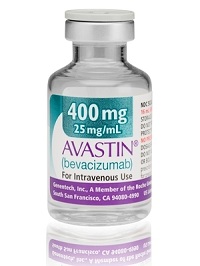
While most of these are supplements for erectile dysfunction or other less dangerous products, the FDA points out that in 2012, counterfeit versions of Roche's ($RHHBY) Avastin and unapproved versions of other cancer drugs manufactured for other countries were found in the U.S., putting cancer patients at high risk. But if a seller appeals, the FDA is bound by current rules to return the products, renewing the cycle. "There is currently little deterrence to prevent sellers from sending violative drugs or resending previously refused drugs into the United States," FDA said in its proposal.
The proposed rule also comes as Europe has discovered an organized crime ring there has been involved in stealing cancer drugs and then reselling them throughout Europe. Some vials regulators retrieved had little or no active ingredient in them.
- read the Regulatory Focus story
- here's the FDA proposal