 |
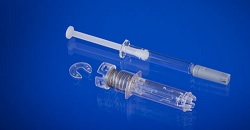
| Novaguard--Courtesy of West |
Exton, PA's West Pharmaceutical Services just announced that the FDA has given it 510(k) clearance for the NovaGuard Staked-needle Automatic safety system, as the push to avoid needlestick injuries gathers momentum.
The company says that the device shields the syringe's exposed needle after it has been activated and contains a tamper-evident safety mechanism to prevent early activation of the device.
According to the World Health Organization, healthcare workers suffer from two million cases of unintentional needlestick injuries a year, potentially resulting in diseases like hepatitis and HIV.
"With increased global emphasis on preventing the spread of blood-borne infectious diseases, there is a need to develop and implement safer systems and practices in healthcare facilities worldwide," said Graham Reynolds, vice president of marketing and innovation, in the release. "This includes adopting devices that can help reduce the risk of infection caused by needlestick. West has deep expertise in needlestick prevention and a portfolio of offerings that enable safe and effective drug administration. It's our hope that the NovaGuard SA safety system is used as a cost-effective solution to help improve needle safety."
Several other companies have efforts underway to prevent needlestick injuries or reduce needle use all together. For example, Portal Instruments, a new company based in Cambridge, MA, secured $11 million for its computerized needle-free drug delivery system via a Series A funding round led by Sanofi ($SNY), Boston-based VC PBJ Capital and a major medical device company. It became only the third company to receive funding under Sanofi's Sunrise Initiative for early-stage companies.
West also announced that it has upped its manufacturing capability in France, the country in which more 1.5 billion similar devices have previously been made. That's in addition to the €100 million ($125.7 million) plant underway in Ireland.
Last year, West rolled out an adapter for standard needles that enables easier and more consistent delivery into a patient's skin at a very shallow angle. The intradermal adapter clips onto the needle and guides the tip into the skin's intradermal space--between the epidermis and the dermis--for shots that include allergy tests and assays for elements such as rabies or tuberculosis.
- read the release