 |
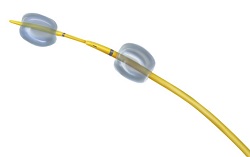
| RenovoCath RC120--Courtesy of RenovoRx |
Many drug-delivery device companies are developing tools to combat peripheral vascular disease, though few mention the visceral vasculature specifically. But the FDA recently gave 510(k) clearance to a two-balloon catheter for the targeted delivery of diagnostic and therapeutic agents to the visceral vascular system. This subset of the peripheral vasculature consists of the arteries that supply blood to the intestines, spleen and liver.
RenovoRx's RenovoCath RC120 catheter consists of ports for both inflation balloons, as well as an infusion port for drug delivery. In addition, it has a two-part handle which enables use of a guidewire to position of the balloon once it is inside the artery, the Menlo Park, CA, company said in a Nov. 4 release.
The device provides physicians with more flexibility to target selected sites in the vasculature than single or fixed balloon catheters, RenovoRx said. "We can now introduce our novel catheter to the clinical market, which will allow physicians to deliver diagnostic and therapeutic agents exactly where they want them," said company CEO Shaun Bagai in a statement.
The RenovoCath is designed for blood flow isolation and delivery of fluids to peripheral arteries, including those of the visceral vasculature system, according to the release. Narrowing of the arteries of the visceral vasculature can result in visceral artery diseases. The most common is a potentially fatal chronic mesenteric ischemia, which is the result of insufficient blood flow to the intestine,according to the Yale School of Medicine.
RenovoRx is also developing a double balloon catheter threaded through the femoral artery to deliver drugs directly to the pancreas for use in pancreatic cancer and diabetes.
The peripheral vasculature refers to blood supplying vessels not of the brain or heart, and includes the visceral arteries. Recently, there has been growing interest in the use of drug-coated balloons to treat peripheral arterial disease.
The RenovoCath does not have drug-coated balloons, but the advent of those devices is still a couple years away in the U.S., where the first one was only just approved by the FDA.
- read the release