 |
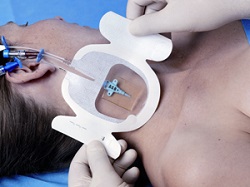
| Tegaderm CHG dressing and gel pad--Courtesy of 3M |
3M ($MMM) announced the launch of its Tegaderm CHG intravenous port dressing, saying the device will help oncology patients receive injections comfortably and ward off infection. The new product combines a transparent port-site dressing with a gel pad for localized drug delivery. 3M says the dressing enables continuous, unobstructed observation of the IV insertion site.
Meanwhile, the gel pad provides antimicrobial protection and conforms around the needle at the insertion site, according to the 3M release. Both parts can be worn for as long as 7 days.
Chlorhexidine gluconate (found in mouthwash) is dissolved within the gel pad and becomes active upon the pad's contact with the skin to provide continuous protection against catheter-related bloodstream infections, 3M says on the product website.
"In the fight against infection, the dressing really matters, and too often patients with implanted venous ports are left with uncomfortable, ill-fitting dressings that don't do enough to ward off contaminants," said Leslie McDonnell, vice president of global marketing in 3M's Critical & Chronic Care Solutions unit, in a statement. "Clinicians have given our Tegaderm CHG IV Port Dressing 90 percent or better satisfaction ratings versus competitor products when it comes to overall performance, patient comfort, wear time, ease of removal and the ability to visualize the insertion area. This new offering, designed especially for patients with implanted venous ports, provides a barrier against several common pathogens known to cause catheter-related bloodstream infections."
Tegaderm will receive Medicare reimbursement, 3M said. A version containing the dressing, but not the gel pad, will be launched in the summer.
3M sells a variety of drug delivery devices or accessories, such a transdermal patches and transdermal patch liners. In addition, the conglomerate has been seeking pharmaceutical partners for its microneedle delivery devices for the administration of biologics.
Last year, 3M joined forces with Delaware's Invion to develop inhaled drugs for inflammatory airway diseases using its pressurized metered dose inhalation technology.
- read the release