 |
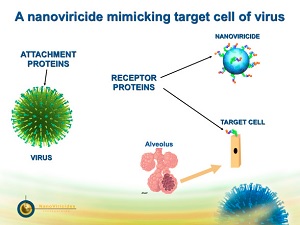
| A nanoviricide mimics a target cell to kill a virus.--Courtesy of NanoViricides |
NanoViricides, a Connecticut-based company named after its own virus-killing technology, is looking to tap into a $40 billion market with its uniquely delivered nanotech designed to "trap" viruses and render them neutral. Most recently, the company's treatment for dengue fever won orphan status from the FDA.
Nanoviricides--the tech, not the company--are polymeric lipids with a virus-binding ligand on the surface. Viruses normally use a ligand to attach themselves to a host cell, where they cause harm. But by exhibiting the ligand on a nano-sized structure, the virus is tricked into attaching to that instead. The nanoviricide then encapsulates the virus and prevents it from infecting cells in the body, according to the company.
And each nanoviricide can be targeted to a certain kind of virus. So far, the company has 5 candidates in preclinical trials for diseases with high commercial value: influenza, HIV, oral and genital herpes in the form of a skin cream, dengue fever and external eye viral diseases in the form of eye drops. NanoViricides also has a rabies treatment in the works, as well as biodefense measures for Ebola and quickly available particles that can be tailored to novel infections.
The recent addition of the company's DengueCide for dengue fever to the list of the FDA's orphan drugs could possibly give NanoViricides a corner on the market. The treatment showed an unprecedented 50% survival rate in animal trials. When left untreated, dengue fever has 100% fatality, and there are only a few drugs in the clinical stage, including balapiravir from Roche ($RHHBY).
"Now we intend to accelerate our dengue drug development programs to take advantage of these benefits," CEO Eugene Seymour said in a statement. The company currently has $16 million in cash and assets, according to a MedCity News report, with hopes to use those funds to bring their influenza candidate to clinical trials.
- here's the release
- and the MedCity News report