Creating the materials used for some of the most intricate nanostructures in drug delivery sometimes requires going back to the basics. In the case of researchers at the University of Oregon and the Berkeley Lab, this meant looking at the interaction between oil and water, developing nanosheets that could be used to compile delivery vehicles down the road.
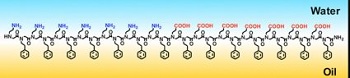 |
| The formation of a peptoid nanosheet between layers of water and oil--Courtesy of UO |
The self-assembling nanosheets are made up of synthetic proteins called peptoids, which are known to be more stable and less likely to break down than natural proteins. And the peptoids can be tailored with precision, co-author Ron Zuckermann of Berkeley said in a news release. To make the ultrathin sheet, the peptoids line up where water and oil meet. Because of this layered interface, they are highly ordered and present a naturally thin but long structure conducive to the creation of sheets.
These peptoid nanosheets are expected to play a role in developing more intricate nanostructures for specifically targeted drug delivery.
"Supramolecular assembly at an oil-water interface is an effective way to produce 2D nanomaterials from peptoids because that interface helps pre-organize the peptoid chains to facilitate their self-interaction," Zuckermann said. "This increased understanding of the peptoid assembly mechanism should enable us to scale-up to produce large quantities, or scale-down, using microfluidics, to screen many different nanosheets for novel functions."
Prior to using oil, the team used the interaction of water and air to form the sheets--essentially, the sheets would float on the water. But with oil, the process could be controlled to a greater extent and include chemical reagents not possible with air.
"We often think of oil on water as something that is environmentally bad when, in fact, my group over the past 20 years has been studying the unique properties of the junction between water and oil as an interesting place for molecules to assemble in unique ways--including for soaps and oil dispersants," co-author Geraldine Richmond of UO said in a statement. "This study shows it is also a unique platform for making nanosheets."
- here's the UO report
- and here's the abstract