 |
| Cryo-electron microscopy image of Arikayce--Courtesy of Insmed |
Insmed's ($INSM) Phase II trial of Arikayce, or inhaled liposomal amikacin for the treatment of nontuberculous mycobacterial lung infections (NTM), did not meet its primary endpoint, but new data released May 20 from the 84-day open-label extension show that patients receiving the compound experienced significant improvement, the company said.
Out of 68 patients who completed both phases of the study, 21 showed no sign of the bacteria.
Investors punished the company in March for failing to meet the primary endpoint during the 84-day double-blind phase, but judging by their positive reaction to the latest news (Insmed stock rose nearly 7%), the candidate and its proprietary liposomal technology for delivery of drugs to the lung is still promising.
Arikayce is delivered in liposomes consisting of a water core containing water-soluble drugs and a hydrophobic chain bilayer interior containing water-insoluble drugs. Arikayce says the liposomes' neutral charge helps them treat infections by resisting attraction to negatively charged compounds on the surface of mucus and protective biofilm.
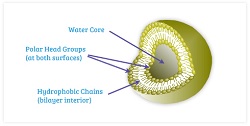 |
| Insmed's liposomal technology--Courtesy of Insmed |
Other advantages include localized delivery and the liposomes' small size, which the company says enables the drug to access bacteria within infected microphages. The technology is also being used in Insmed's Phase III candidate for the treatment of cystic fibrosis.
Arikayce is inhaled using an investigational nebulizer developed by PARI Pharma.
The study of Arikayce for the treatment of NTM consisted of an 84-day double-blind portion comparing the standard of care for NTM plus placebo to the standard of care plus Arikayce. It was followed by an 84-day open-label phase in which all subjects received Arikayce as a supplement to the standard of care. Patients in the trial were on standard care unsuccessfully for at least 6 months, according to the statement.
Arikayce did not demonstrate statistical significance for the measurement of the change in mycobacterial density on a 7-point scale by the end of the double-blind phase of the trial. The trial resulted in a p-value of 0.148 for that measure, short of the prespecified target, according to Insmed's statement. However, the trial did meet its secondary endpoint for the proportion of subjects with culture conversion to negative.
It will be interesting to watch how FDA interprets the mixed results from the trial. It has given the candidate Orphan Drug, Qualified Infectious Disease Product and Fast Track designations for the treatment of NTM.
- read the release
- view the poster presented at American Thoracic Society meeting (PDF)
Related Articles:
Insmed faces another setback as lead therapy flunks PhII lung study
Insmed shares routed on head-to-head antibiotic Phase III results
Insmed stock soars after FDA ends hold on key lung drug