 |
| The ND0612L patch pump for moderate Parkinson's--Courtesy of NeuroDerm |
Investors were thrilled by the Phase IIa clinical trial results of NeuroDerm's ($NDRM) continuously delivered Parkinson's candidates that are administered via a belt or patch pump, offering patients an alternative to traditional orally delivered levodopa, which is notorious for its fluctuating concentrations in the blood.
The company's stock price more than doubled to $18 upon the year-end news of the positive results. The shares now sell for about $15.50 on the Nasdaq, but that's still above last year's IPO price of $10 and its December 2014 low of $6.
The company reported that the two candidates, ND0612L and ND0612H, demonstrated safety and were tolerated by the patients. Moreover, the fluctuations in levodopa plasma levels were lower compared to that achieved by oral administration of the drug. For that feat, thank the candidates' novel form of continuous drug delivery, which is designed to overcome levodopa's short-half life and provide a steady level of the drug in the body.
ND0612L, for moderate Parkinson's, is delivered via a small belt-worn pump similar to that used to provide insulin to diabetics, or a patch pump, NeuroDerm explains on its website. ND0612H is for severe cases and is only available via belt pump. All modes of administration are subcutaneous forms of delivery.
Using approved therapies, continuous delivery of levodopa can only be achieved by an invasive surgery to permanently implant a tube into the duodenum (the upper part of the small intestine), NeuroDerm says.
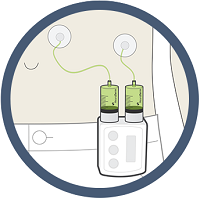 |
| The ND0612H belt pump for severe Parkinson's--Courtesy of NeuroDerm |
"These results add to the growing body of clinical data confirming our thesis that continuous, subcutaneous delivery of LD/CD leads to more consistent therapy, which we expect to have a dramatic effect on patient outcomes and quality of life, replacing in most cases the need for surgical intervention," said NeuroDerm CEO Oded S. Lieberman in a statement. "Based on these positive PK results, we will proceed with the clinical development of ND0612H and ND0612L in the United States and the European Union in 2015."
Jefferies & Co. analyst Thomas Wei told Reuters that NeuroDerm's candidates appear safer or more convenient than competing levodopa-based candidates for Parkinson's that are being developed by Impax Laboratories ($IPXL) and AbbVie ($ABBV). The FDA is expected to approve (or reject) those therapies this year, while NeuroDerm hopes to achieve U.S. or European approval in 2018, according to an SEC filing.
Civitas is also scrambling to develop an alternative drug delivery method for levodopa. The Chelsea, MA-based biotech is developing CVT-301, a fast-acting rescue formulation of levodopa that can be self-administered through an inhaler.
- read the release
- here's the Reuters' story
Related Articles:
NeuroDerm soars on promising Parkinson's results
NeuroDerm preps for Parkinson's patch PhII trial
Civitas, NeuroDerm, EyeGate to pursue IPO dreams, combined $165M on the line
Civitas announces positive results from Phase II inhaled levodopa trial