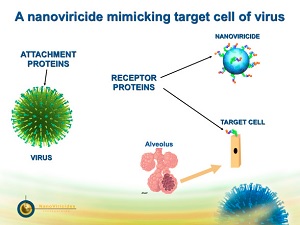
 |
| A nanoviricide mimics a target cell to kill a virus.--Courtesy of NanoViricides |
Connecticut-based NanoViricides ($NNVC), with its novel antiviral nanotech delivery, pulled in $20 million in an offering from a combination of unnamed institutional investors and existing shareholders.
With now $40 million in hand, the company hopes to push its FluCide influenza treatment forward through early- and mid-stage trials and bring DengueCide into the clinic. Its Dengue candidate won orphan status from the FDA last summer.
NanoViricides' treatments are all based on the same technology, which uses nanoparticles with ligands on their surface that attract viruses, allowing the particle to encapsulate the virus and prevent it from infecting cells in the body. Along with its lead candidates, the company hopes to advance its HIV drug and ocular herpes drug into early trials, as well.
"All of our drugs consist of two parts," CEO Eugene Seymour told FierceDrugDelivery. "We have a nanomicelle made of biodegradable nontoxic polymers and then, to make the drug unique for a particular virus, what we do is we mimic the protein that's expressed on the surface of that particular host or target cell."
And with this mix-and-match kind of deliverability, it can take as few as a couple days to get a drug out in the open, Seymour said.
If approved, FluCide would have Tamiflu to contend with. Roche's ($RHHBY) liquid flu fighter gave that company $335 million in sales last year when the virus was especially tough. This showed 84% growth for the drug. NanoViricides is betting its one-tech solution can give it a leg up against a giant like Roche by grabbing several segments of the antiviral market at once.
"Say I'm in Rwanda and I need a thousand doses of ebola," Seymour said. "We have that mimic protein in our library, we take that from our library, attach it to the nanomicelle out of the stockpile, and within a very short time, we have a drug."
So with a part of the new funding, NanoViricides will also begin scaling up its manufacturing process while at the same time performing toxicology studies for its strands in preparation to submit to the FDA. The company has plans to head to Australia for its initial trials.
According to its annual report filed in October, the total estimated market size for NanoViricides' candidates is over $40 billion, and potentially more depending on the success of its anti-influenza drug. With the overnight sale of almost four million shares, NanoViricides' stock took a slide, and its ability to wrangle a part of the huge market will hang on its entire line of candidates.
Among its development products, it has injectables designed to treat epidemic influenza H1N1, H5N1 and avian influenzas; an HIV treatment; eye drops for conjunctivitis and keratitis; drugs for oral and genital herpes; and its dengue candidate. In early research, it has programs for the MERS CoV virus, rabies, ebola/Marburg and other viral hemorrhagic fevers, according to the report.
"This is going to be a major lifesaver," Seymour said. "For flu, I suspect there are a million people hospitalized between the U.S. and the EU, and about 200,000 people die."
The company is now looking to hire, Seymour said, "as many people as we can find" to get its candidates submitted to the FDA as soon as possible.
- here's the release