 |
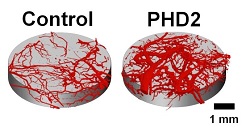
| Side-by-side comparison of impeded blood vessels and those with sustained delivery of siRNA treatment--Courtesy of Craig Duvall, Ph.D., Vanderbilt University |
Researchers at Vanderbilt University have developed a biodegradable network of nanoparticles that can deliver small bits of genetic material and promote blood vessel growth and tissue regeneration.
The delivery of small interfering RNA (siRNA) to cells is difficult and must be done locally to sustain the effect of the gene treatment. In the study, published on December 16, 2013, in the journal Advanced Materials, scientists reported that their tissue scaffold can deliver siRNA to cells in a particular area for several weeks at a time.
The team packaged the genetic material into nanoparticles to protect the fragile strands of siRNA, as they denature very quickly under normal conditions. By adding the compound trehalose, a substance made in nature by weevils and other animals, the nanoparticles could be incorporated into a scaffold that could be implanted under the skin, according to a report from the National Institute of Biomedical Imaging and Bioengineering. With the pore-creating trehalose in tow, the nanoparticles could be released at a variety of speeds from all at once over several weeks.
"Depending on the target gene, and its time course of expression during the wound healing process, you may want to have a fast, short-lived silencing effect, or you may want to have a long-term, sustained silencing effect," said lead author Craig Duvall in a statement. "For example, in a hard-to-close wound, you may want to deliver siRNA for a month, or even longer. With this scaffold, we can make that adjustment by simply tuning the quantity of trehalose."
In a mouse implanted with the scaffold, the researchers aimed to knock down the gene PHD2, which inhibits the growth of blood vessels. By delivering the siRNA for this gene knockdown over 33 days with the scaffold, the mouse shows a threefold increase in local blood vessel volume.
"What we've developed is a platform technology," Duvall said. "One of the nice things about siRNA is that you can design it with high specificity against any target you want. That opens up a whole universe of opportunities in terms of therapeutic intervention both for wounds and for other types of pathological and tissue regenerative applications."
The team hopes to bring it to the clinic in about 5 years.
- here's the National Institute of Biomedical Imaging and Bioengineering report
- get the journal abstract