 |
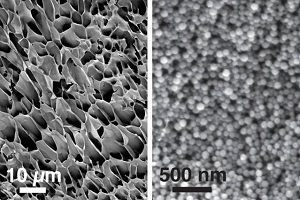
| Scanning electron microscope images of the hydrogel structure at lower (left) and higher (right) magnifications--Courtesy of MIT |
A team of MIT scientists has designed a new injectable hydrogel made from nanoparticles that could someday be used to hold and release drugs in the body, treating diseases such as cancer, macular degeneration and heart disease.
The substance consists of many small nanoparticles structured into a network that is held together by a polymer, in this case cellulose. What's important about this particular structure is that it can be squeezed into the body through a needle and then reform itself, or "re-heal," to once again become structurally sound.
By surviving these mechanical forces, the gel can act as a sort of sponge for as many as two drugs at a time, carrying them within either the nanoparticles or the polymer scaffold. This could allow for a long-term delivery solution, as shown in animal studies--two drugs survived injection under the skin in mice, to be released over several days.
The MIT team published findings in the journal Nature Communications. Famed MIT professor and entrepreneur Robert Langer is listed as the paper's senior author.
"Now you have a gel that can change shape when you apply stress to it, and then, importantly, it can re-heal when you relax those forces," lead author Mark Tibbitt said in a statement. "That allows you to squeeze it through a syringe or a needle and get it into the body without surgery."
Currently the scientists are looking at using the gel to treat macular degeneration using anti-angiogenesis drugs in the eye. Unlike a solution, the gel would allow the drugs to remain there for several months, negating the need for frequent, damage-causing injections to the eye.
They are also looking to combine the gel with a surgical procedure for cancer, filling the spot of a removed tumor with a drug-delivering hydrogel sponge.
"We're working with really simple materials," Tibbitt said. "They don't require any advanced chemical functionalization."
- here's the MIT report