 |
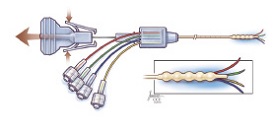
| The investigational delivery catheter in its deployed state--Courtesy of Cleveland Clinic |
Cleveland Clinic's investigational convection-enhanced catheter for the delivery of drugs directly into the brain showed promise in first-in-human clinical trials of brain cancer patients.
Using infusion, the so-called Cleveland Multiport Catheter successfully delivered the chemotherapy drug topotecan into the brain using conventional neurosurgical techniques, Cleveland Clinic says.
It consists of four microcatheters that are deployed from the wall of a central catheter implanted in the brain. Other convection-enhanced delivery catheters have only a single port and require intraoperative imaging. They can only be implanted for several hours, compared to several days for the Cleveland Multiport Catheter, the Cleveland Clinic says.
The device was developed by the clinic's department of biomedical engineering, with the help of multinational engineering company Parker Hannifin. The drug/device combination product is being regulated under the FDA's drug pathway, which is generally slower than the device one.
Clinical trials to optimize drug delivery by finding the best rate and duration of infusion are ongoing, led by Cleveland Clinic spinoff Infuseon Therapeutics. In time, the company hopes to partner with a Big Pharma or a biotech player on drugs that require delivery directly to the brain.
Implantable delivery devices offer a way to work around the blood-brain barrier, which has so far proved insurmountable to oral meds and injectables. Flowonix is also developing implantable infusion-based devices for delivery directly into the brain under a just-announced drug delivery partnership with Cerebral Therapeutics aimed at treating neurological diseases like epilepsy.
Meanwhile, Impel Neuropharma believes its Precision Olfactory Delivery device exploits a weakness of the barrier because the nose-to-brain pathway apparently lacks a significant membrane barrier. 3M recently agreed to help Impel develop the device.
- read more from the Cleveland Clinic
- here's more about the clinical trial from ClinicalTrials.Gov