 |
| Courtesy of BioDelivery Sciences |
BioDelivery Sciences ($BDSI) is looking at a possible $60 million to boost programs in its lineup including the launch of its opioid dependence film Bunavail and continue on the approval path for its pain gel for diabetic neuropathy.
With funds from the planned direct offering--led by investor Federated Kaufmann with 7.5 million shares on the line--BioDelivery Sciences signed the deal to ramp up to the launch of its Bunavail opioid dependence oral film in particular, preparing for a June 7 FDA decision in the works.
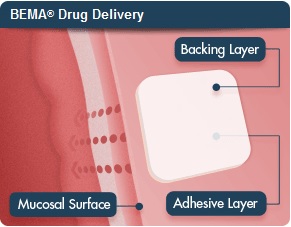
Bunavail makes use of the company's own Bema delivery technology, which is a bioerodible film placed on the inner lining of the cheek that quickly delivers a dose of the drug buprenorphine/naloxone across the mucous membrane. It's designed to dissolve within 15 to 30 minutes, according to the company.
The Raleigh, NC, company is also looking to advance its Phase III pain gel with the new funding. The topical gel, designed to treat symptoms of painful diabetic neuropathy, delivers the drug Clonidine, which stimulates a receptor subtype associated with pain receptors in the skin.
"Articles suggest patients can realistically expect a 30% to 50% reduction in discomfort with available products at their maximal doses," vice president of marketing Al Medwar told FierceDrugDelivery in December, when the company announced it would be moving forward after a positive meeting with U.S. regulators. The FDA confirmed fast-track designation for the program, which the company hopes will lead to a priority review.
Along with these two programs, the company will also be looking to acquire additional products for its pipeline, according to the release, focusing on the central nervous system, addiction and pain.
BioDelivery Sciences partnered with Endo Pharmaceuticals ($ENDP) in a $20 million deal last year to commercialize its BEMA Buprenorphine film, another pain treatment using the delivery technology. The program is currently in Phase III trials.
- here's the release