 |
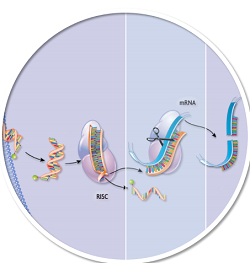
| An illustration of the RNAi pathway by which targeted genes are silenced using small interfering RNA. The picture features the RNA-induced silencing complex and messenger RNA, which are critical components of the naturally occurring process.--Courtesy of Alnylam |
Alnylam ($ALNY) and Isis Pharmaceuticals ($ISIS) recently stole a page from the bacterial playbook, announcing an intellectual property cross-licensing agreement, akin to the strategy of horizontal gene transfer, whereby different species of bacteria swap genes in order to build up antibiotic resistance. Similarly, the two companies hope their IP swap leaves them both better equipped to deal with the challenges of developing the first commercial RNAi therapy, including utilization of the promising but still-unproven RNA interference drug delivery pathway, and sometimes jittery investors.
The deal centers on four therapeutic areas, and builds on an alliance formed in 2004. Each company will receive exclusive licensing rights to the other's IP in two of its R&D programs in return for royalty payments.
Alnylam gains exclusive rights to Isis's IP for therapeutics targeting the proteins antithrombin (AT) and minolevulinic acid synthase-1 (ALAS-1). Alnylam's Phase I program to treat the bleeding disorder hemophilia targets AT. And the company just filed an application to begin Phase I trials of its ALAS-1 targeting treatment for hepatic porphyrias (a class of rare inherited disorders that are the result of deficiencies in some enzymes of the liver), according to the release.
 |
| Alnylam CEO John Maraganore |
"We believe that our collaboration has enabled both companies to succeed as leaders in the development and commercialization of RNA therapeutics," said Alnylam CEO John Maraganore, in the statement announcing the agreement. "This extended agreement adds a new feature, enabling further advancement of specific therapeutic programs on an exclusive basis, while allowing for the continued sharing of IP on technology. Based on the benefit that we believe innovative RNA therapeutics may bring to patients across a broad range of diseases, we look forward to continued success for both companies."
Meanwhile, Isis receives exclusive rights to Alnylam's IP for therapeutics against the blood clotting Factor XI and apolipoprotein (a), which is a protein component of high density lipoprotein (also known as HDL or "good" cholesterol). Isis' anti-blood clot candidate targeting Factor XI is in Phase II, as is its apolipoprotein (a)-targeting candidate for cardiovascular disease, according to the release.
"Since we began our collaborative efforts in 2004, Alnylam has been a great partner and has made excellent progress in its RNAi therapeutic programs. This expansion of our license agreement will enable both companies to enhance drug development efforts in particular disease areas while also sharing IP access," said Isis CEO Dr. Stanley T. Crooke in the statement.
There is also a nonexclusive cross-licensing component to the agreement. The release states that "Alnylam is granting Isis a royalty-bearing, nonexclusive license to new platform technology arising from May 2014 through April 2019 for single-stranded antisense therapeutics. In turn, Isis is granting Alnylam a royalty-bearing, nonexclusive license to new platform technology arising from May 2014 through April 2019 for double-stranded RNAi therapeutics."
- read the release