A drug combination from Johnson & Johnson outperformed AstraZeneca’s Tagrisso in slowing the worsening of newly diagnosed lung cancer. But it’ll take more than a tumor progression win to dethrone the EGFR king.
In a high-stakes, head-to-head trial, J&J’s Rybrevant and the company’s Yuhan-partnered lazertinib reduced the risk of progression or death by 30% versus Tagrisso in patients with newly diagnosed, EGFR-mutant non-small cell lung cancer (NSCLC).
Compared with the Tagrisso group, the Rybrevant-lazertinib regimen improved the median time patients lived without disease progression by 7.1 months to reach 23.7 months.
The data, from the closely watched MARIPOSA trial, were presented during a presidential session at the European Society for Medical Oncology 2023 congress.
The trial establishes Rybrevant and lazertinib as a new first-line standard of care for EGFR-mutated advanced NSCLC, the trial investigators wrote in an abstract. In an interview with Fierce Pharma, Mark Wildgust, Ph.D., VP of oncology global medical affairs at J&J’s Janssen, also referred to Tagrisso as “the former standard of care.”
Commercially, it's a big deal for the J&J combo to be able to challenge Tagrisso. Last year, the well-entrenched EGFR inhibitor reeled in $5.4 billion in sales.
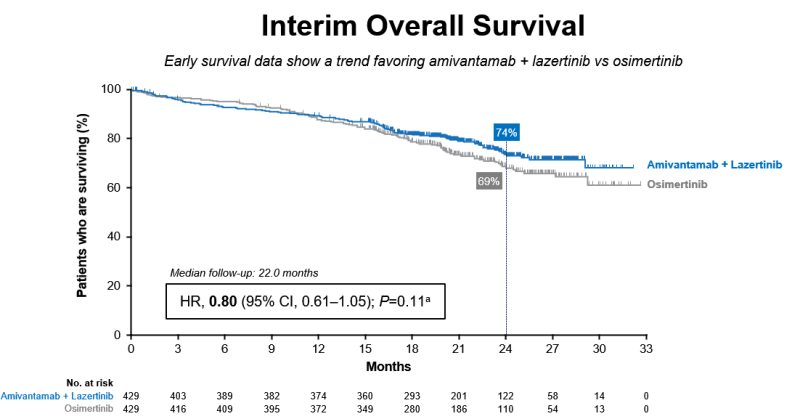
Key overall survival data
Still, it's an open question whether J&J can steal meaningful market share from AZ. As Leerink Partners analyst Andrew Berens, M.D., noted during an interview with Fierce Pharma before the MARIPOSA data release, doctors and patients like Tagrisso not only because of its ability to stall cancer progression.
“I think the real question that doctors are going to ask when they decide to prescribe [Rybrevant and lazertinib] or not is, is it improving overall survival?” Berens said.
A better progression-free survival showing could be expected from the combo given its mechanism of action, Berens explained. Lazertinib, like Tagrisso, is a small-molecule EGFR inhibitor. Rybrevant is an EGFRxMET bispecific antibody, with MET known as an EGFR escape pathway.
For now, the J&J combo showed an encouraging trend toward further extending patients’ lives. The cocktail reduced the risk of death by 20% over Tagrisso. Although the number hasn’t reached statistical significance, J&J’s Wildgust said that based on the current trend, J&J would anticipate “the potential to see a significant difference” in overall survival in the future.
A graphic presented at ESMO showed that the Rybrevant-lazertinib combo began to lead AZ's drug on patient survival starting at about 12 months. The two survival curves continued to stay separated after that time point.
Wildgust also pointed to data on disease progression following a subsequent therapy, an endpoint known as PFS2, which he said is strongly correlated with overall survival. There, the J&J combo reduced the risk of second disease progression or death by 25% over Tagrisso.
A progression-free survival comparison
But even on the PFS measurement, Rybrevant and lazertinib don’t boast the most impressive data in this patient group.
Before the MARIPOSA readout, another phase 3 trial called FLAURA2 showed that Tagrisso’s combination with chemotherapy could cut the risk of progression or death by 38% over Tagrisso monotherapy in first-line, EGFR-mutated NSCLC. Adding chemo to Tagrisso prolonged the median PFS by about nine months.
Wildgust argued that the two trials are different, partly because MARIPOSA followed patients with brain metastases more closely and was therefore “looking even harder for progression.”
In a separate analysis that only measures progression outside the brain, the J&J combo reduced the risk of extracranial progression or death by 32% and improved median PFS by nine months.
In a note to clients last week, Leerink’s Berens argued that because MARIPOSA is proposing a chemo-free regimen, putting the PFS data from MARIPOSA and FLAURA2 side by side would be an apples-to-oranges comparison.
During his interview with Fierce Pharma, Berens said that in investors’ minds, the FLAURA2 readout raised the PFS efficacy bar for Rybrevant and lazertinib to around nine months, which the MARIPOSA regimen now apparently failed to meet. Still, doctors care most about overall survival, Berens said.
For now, the J&J combo’s 20% death risk reduction looks better than FLAURA2’s 10% improvement over Tagrisso alone. In FLAURA2, the survival curve of the Tagrisso-chemo arm struggled to separate from that of the monotherapy.
On J&J’s third-quarter earnings call last week, the company’s pharma R&D chief John Reed argued that chemo was “best reserved” for when patients have failed on front-line targeted therapies, noting that almost all lung cancer patients will eventually relapse. Wildgust, in his interview, echoed that sentiment with the question for a chemo-containing regimen, “what do you use afterwards?”
“When you look at trying to change standard of care, we should be looking to use novel therapies to be able to do that,” Wildgust said. “We believe that Rybrevant’s immune-mediated mechanism of action […] really provides a novel innovative approach that’s really going to benefit patients.”
Then there’s the question: Will doctors reserve Rybrevant and lazertinib for second-line treatment, so they would have another option following progression on Tagrisso? The phase 3 MARIPOSA-2 trial tested that scenario, and its data were also presented at ESMO 2023.
Compared with chemotherapy alone, adding Rybrevant reduced the risk of progression or death in Tagrisso-progressed patients by 52%, and the Rybrevant-lazertinib-chemo triplet cut the same risk by 56%, according to calculations from a blinded review.
As for overall survival, the doublet reduced the risk of death by 23% at an interim analysis. The triplet didn’t show any overall survival benefit. The triplet regimen was modified during the study after an independent data monitoring committee noted increased hematologic toxicities with the original version. The data include all randomized patients regardless of dosing regimen received.
The MARIPOSA-2 trial showed that Rybrevant can be that second-line treatment for patients who progressed on Tagrisso, Wildgust said. But now that the Rybrevant-lazertinib combo has beaten Tagrisso, the AZ drug is “really the old standard of care now," he said.
“A quarter of patients will die between first line and second line,” Wildgust said. “The idea of using your best drug second when you know a quarter of patients will not make it from first to second line is really not the best clinical thing for patients.”
Beyond efficacy
Despite FLAURA2’s big showing in PFS, AZ said it still believes Tagrisso monotherapy will remain the standard of care for the majority of EGFR-mutant NSCLC patients. For J&J, a potential overall survival win matters because of Tagrisso’s favorable convenience and tolerability.
Tagrisso is an oral drug with a very benign side effect profile, Berens noted. By comparison, Rybrevant is an infusion currently approved by the FDA to treat a small subset of the NSCLC marked by EGFR exon 20 insertion mutations.
Frequent infusions and toxicities have made Rybrevant a difficult drug to take for some patients, the Leerink analyst said.
In MARIPOSA, grade 3 or above side effects occurred in 75% of patients who got the Rybrevant-lazertinib combo, versus 43% for Tagrisso. Side effects led to discontinuation of any agent in 35% of the combo arm, compared with 14% in the Tagrisso group. Adverse events led to deaths in 8% and 7% of patients in the two arms, respectively.
Wildgust suggested that if approved, the J&J regimen’s tolerability profile could improve in the real world as doctors learn to manage some of the side effects up front. As for convenience, J&J is working on a subcutaneous version of Rybrevant.
Still, Wildgust believes doctors will pick their first-line treatment based on efficacy. And Leerink’s Berens also said that overall survival data is key.
It would be beneficial to have a few extra months before the tumor started growing again, Berens said. “But at the end of the day, if it doesn’t improve OS, I think patients are going to opt for the quality of life.”
Editor's Note: This story was updated to include new information from the Rybrevant studies and an interview with J&J's Mark Wildgust. The story was originally published on October 17 at 3:44 p.m. ET.