Even though the BCMA CAR-T therapies Carvykti and Abecma showed a potential problem of early patient deaths in their separate trials, experts on the FDA’s Oncologic Drugs Advisory Committee (ODAC) still believe that the drugs’ long-term benefits outweigh their risks.
Friday, the 11 experts voted unanimously in favor of Carvykti as a treatment for multiple myeloma patients who’ve tried one to three prior lines of therapy. The Johnson & Johnson and Legend Biotech CAR-T is currently approved as a fifth-line therapy.
The panel was not in complete agreement on Abecma. The same experts voted 8-3 that Abecma’s data support a positive risk-benefit profile for the Bristol Myers Squibb drug when used in third- to fifth-line myeloma.
The FDA called Friday’s ODAC meeting because the agency noticed that patients who received the CAR-T drugs were more likely to die than those who received standard drug combinations early in their respective trials.
In Carvykti's case, the agency questioned whether an increased death risk in the first 10 months of the CARTITUDE-4 study could outweigh the 59% reduction in the risk of disease progression or death—and an interim 43% reduction in the risk of death—that Carvykti has shown in the trial.
Abecma faces the same early-death question. In the KarMMa-3 trial, the FDA noticed a detriment to patient survival up to Month 15 after randomization. But unlike Carvykti’s trial, Abecma hasn't shown any life extension benefit—so far—despite delivering a 51% reduction in the risk of progression or death.
During Friday’s deliberations, the FDA mainly raised concerns that the delay in getting patients onto treatment and toxicity may have contributed to the early deaths.
The fact that some patients died while waiting to receive Carvykti is an important factor in those trial deaths, an FDA official pointed out during the drug's review session. This pattern “raises issues in the subject selection [and] optimal disease control while awaiting CAR-T product,” Helkha Peredo-Pinto, M.D., a clinical reviewer at the FDA, said during the meeting.
While the survival advantage swung in Carvykti’s favor after 10 months of the study, J&J noted that the increased death risk was essentially limited to the first three months. During that period, seven patients in the Carvykti patients passed away, versus just one in the control group. For every three-month period after that, the number of deaths in the Carvykti arm was either the same or lower than the control group.
J&J’s analysis pointed out that the 32 patients who progressed or died before receiving Carvyti indeed drove that early survival red flag. But the company suggested that a suboptimal bridging therapy—not the waiting itself—could be to blame for those early progressors.
J&J also agreed with the FDA that there’s currently no good method to identify at-risk patients for potential enhanced bridging management. The New Jersey pharma hypothesized that improving bridging therapy could mitigate the early death risk, but the FDA argued that the company has a responsibility to prove that point rather than asking the agency to rely on a theory.
Despite the FDA’s concerns, the ODAC experts agreed that the agency’s attention shouldn’t be fixated on those few initial events that don’t bear statistical significance. Instead, the risk should be viewed in the context of Carvykti’s statistically significant benefits over a longer timeframe.
“When you look at the context of the harm and a handful of events in the early months, compared to the tail of the curves that we’re seeing, I think it’s a pretty clear signal of benefit,” Christopher Lieu, M.D., from the University of Colorado, said during the meeting.
As at least two experts noted, signs of an early survival detriment in an overall positive trial aren’t completely unheard of in cancer drug development. One FDA staffer agreed that Carvykti’s overall survival pattern looks like just a stem cell transplant, in which physicians ask patients to accept an upfront burden of increased risk of death because there’s clear evidence of an overall survival benefit down the line.
But in this case, the FDA was just not sure whether that later benefit warrants the initial risks.
Still, the ODAC members were convinced on J&J and Legend’s application based on CARTITUDE-4’s striking progression-survival showing and positive overall survival data, which were about half complete before a final analysis. The overall survival curves show that Carvykti’s advantage keeps widening as the trial progresses.
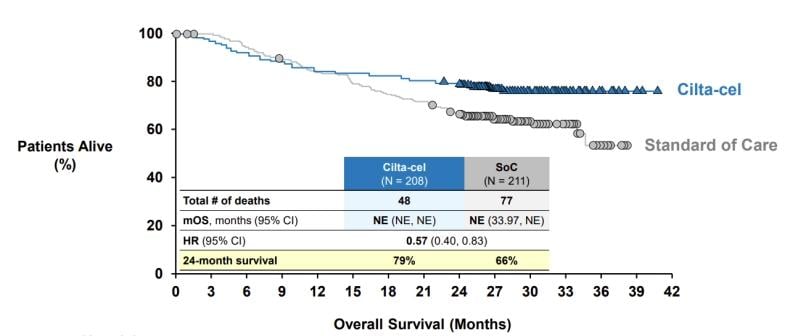
“I felt that the long-term PFS was compelling, [and] the survival is going in that direction,” William Gradishar, M.D., from Northwestern University, said. “Furthermore, the upfront risk, I remain unconvinced that that’s directly attributable to the therapy itself.”
As for Abecma, several members on the ODAC panel expressed concerns of a lack of “flattening” of the drug’s PFS curve down the road, which would have indicated a longer-term benefit that some patients may have been cured.
Daniel Spratt, M.D., from Case Western Reserve University, was among those who weren't satisfied with Abemca’s longer PFS performance. On the topic of moving the drug up in treatment sequences, he said “there’s no clear benefit that earlier is better than later,” because control patients who progressed and later received the drug experienced a favorable overall survival trend.
Since patients appeared to be able to benefit from Abecma later in their treatment journey, along with the increased rate of deaths early in the study, Spratt figured there remain uncertainties around moving Abecma into earlier lines of treatment.
Still, the majority of the experts stuck to the trial’s primary endpoint of PFS, where Abecma showed a large benefit.
Stanford University's Ranjana Advani, M.D., labeled Abecma's PFS data as “very convincing.” She agreed that the early deaths were related to patients who had a rapidly progressive disease and didn't get Abecma in time.
Sally Hunsberger, Ph.D., a statistician with the National Institutes of Health, agreed with BMS that KarMMa-3 was underpowered for an overall survival analysis because of the crossovers and that the data set was uninterpretable.
“I think the PFS drove my decision,” Hunsberger said. “I think there is still the bridging question and how to really implement that. But I think the study was designed for PFS and the overall survival is—it's not harmful as far as I can see.”
The FDA is set to deliver a verdict on J&J and Legend’s application for Caryvkti in earlier-line myeloma by April 5. The agency’s previously scheduled target decision date for Abecma passed in December.
The agency doesn’t have to follow the opinions of advisory committees, but it typically does.
Editor's note: The story has been updated with the Abecma portion of the ODAC meeting.