A federal indictment alleges that the now-defunct New England Compounding Center reaped millions of dollars by cutting corners in the manufacture of what were supposed to be sterile drugs, falsifying records, using expired ingredients and lying to clients about their safety. The result was that drugs became contaminated with fungal meningitis, infecting hundreds and killing 64 people. Now federal prosecutors say 14 NECC employees, from a majority shareholder to technicians, need to pay the consequences, and that might include going to jail.
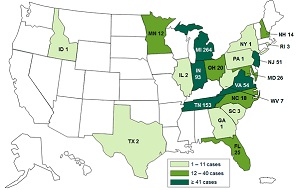 |
| A CDC case count map for the 2012 meningitis outbreak (Click for a larger version) |
The allegations were laid out in a 73-page indictment handed up by a federal grand jury on Wednesday in Massachusetts, the Department of Justice (DOJ) announced on Wednesday. Among the allegations is that NECC often cut short the autoclaving of drugs, slighted sterility testing and when tests did find microbial growth, the company did not recall suspect drugs. The result was production of a steroid that was tainted by fungus, sickening patients who had it injected to treat their pain.
"As alleged in the indictment, these employees knew they were producing their medication in an unsafe manner and in insanitary conditions, and authorized it to be shipped out anyway, with fatal results," said Attorney General Eric Holder in a DOJ release.
Eleven of the 14 were arrested Wednesday, Bloomberg reported. Among those indicted are Greg, Douglas and Carla Conigliaro, shareholders of the company; Gene Svirskiy, a supervising pharmacist in one of the "clean rooms" and Robert Ronzio, the company's national sales director. Barry Cadden, NECC's former president and lead pharmacist, and Glenn Chin, a supervising pharmacist who was arrested at a Boston airport in September before leaving the country, have been accused of second-degree murder in the deaths and face up to life in prison.
The 2012 outbreak shined a spotlight on the growth of the drug compounding industry, where large operations were mass-producing drugs and using sales forces to market them nationally. The outbreak raised serious questions about why the FDA was not inspecting most compounding facilities and led to Congress passing the Drug Quality and Security Act, which gives the FDA new, but limited, powers to oversee compounding pharmacies that volunteer to be regulated. While it did not get the authority it sought, the agency has been aggressive with violators and this month elicited a guilty plea to a federal criminal misdemeanor from the co-owner of Main Street Family Pharmacy, a Tennessee-based compounding pharmacy. The case was tied to its sales of a contaminated steroid that caused 26 people to become ill.
NECC was shut down in October 2012 after authorities traced the outbreak to its facility that produced a tainted steroid, which was distributed to health providers in 20 states. It declared bankruptcy two months later. Earlier this month, a new plan was filed with a bankruptcy court to establish a fund of at least $135 million to compensate victims and families affected by the outbreak.
- here's the DOJ release
- here's the indictment
- read the Bloomberg story