 |
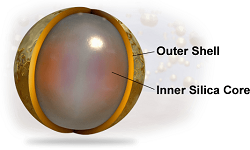
| Sebashell--Courtesy of Sebacia |
Sebacia, the maker of an investigational microparticle-based therapy for acne that's heated by a hand-held laser, received $8 million in financing, increasing its total fundraising haul to more than $40 million.
The company's management includes members of various healthcare financiers, including Accuitive Medical Ventures, Domain Associates and the Redmile Group.
Sebacia's topical cream is applied to the face and then wiped off in an office procedure. The patented microparticles then travel into the pores and sebaceous glands, where they settle. Next, the dermatologist applies a laser to the face, creating a photothermal effect in the follicles, according to the company website.
Sebacia says the delivery paradigm attacks acne by altering the sebaceous glands. Pain is limited because the heating is selective and only affects the structures under the skin where the microparticles settle, the company says.
The company's Sebashell microparticles consist of gold wrapped around a silica core. They are designed to convert the light from the laser into heat.
In March, the Duluth, GA-based company said a study show that the therapy improved inflammatory acne compared to a sham treatment. Patients received three treatments with the system one to two weeks apart and saw results as early as four weeks after treatment concluded, according to a Sebacia statement. The findings were published in the Journal of Investigative Dermatology.
Sebacia is conducting an ongoing, 300-person clinical trial of its acne treatment. The pivotal study ends in July, after which the company intends to file for FDA approval.
There is $3 million of unsold equity remaining in Sebacia's funding round. The company has been an ambitious fundraiser in the past, and SEC filings show that in spite of raising $40 million-plus, it hasn't sold all the equity on offer in its last four rounds.
- here's the SEC filing