 |
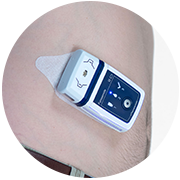
| The Sc2Wear pump--Courtesy of scPharmaceuticals |
Lexington, MA's scPharmaceuticals aims to eliminate use of intravenous peripherally inserted central catheters for the delivery of a common antibiotic made by Roche's ($RHHBY) F. Hoffmann-La Roche AG--and the associated risks of infection, deep vein thrombosis and irregular heartbeat.
To that end, the company touted a promising 18-person, clinical trial of its combination product to administer standard ceftriaxone subcutaneously using its sc2Wear Pump.
On a daily regimen, plasma levels in the treatment group were about 30% higher than in those who received the drug via standard intravenous administration, according to a release. The data will support the company's 505(b)(2) New Drug Application, to be submitted in the second half of the year.
"This represents an important milestone in our quest to make widely-used antibiotics available without the risks, discomfort and costs associated with infusion lines that are routinely required for longer courses of ceftriaxone treatment, said scPharmaceuticals CEO Dr. Pieter Muntendam in a statement. "These results indicate that antimicrobial coverage following subcutaneous administration of ceftriaxone is fully comparable to antimicrobial coverage following standard intravenous administration."
The sc2Wear Pump consists of an activator that controls the pump and a wearable, single-use cartridge. The cartridge contains a micropiston pump to deliver the drug, a needle insertion and retraction mechanism, a fluid reservoir and a base plate with an adhesive backing to adhere to the skin during use, according to the company website.
The micropiston pump, developed by Swiss company Sensile Medical, can pump fluid either upward or downward, enabling it to fill the reservoir directly from a standard drug container in preparation for subcutaneous administration.
Marketed as Rocephin, ceftriaxone is used to treat a variety of bacterial infections and dosed more than 30 million times per year in the U.S. alone, according to scPharmaceuticals.
The company is also developing subcutaneously delivered furosemide to treat fluid overload in heart failure via the Sc2Wear pump, with U.S. and EU regulatory filings expected later this year. The company said that it has developed a novel, proprietary formulation of the drug for subcutaneous delivery.
- read the release