 |
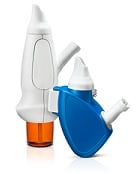
| OptiNose's powder delivery devices with nosepiece and mouthpiece--Courtesy of OptiNose |
Pennsylvania-based OptiNose, the creator of a bidirectional device for nasal medications, garnered positive results in a late-stage trial for its nasal polyp treatment. The company adds the anti-inflammatory treatment to its clinical lineup of migraine and autism candidates, both of which use the same delivery tech.
The nasal polyp treatment, OPN-375, showed a reduction in nasal congestion and obstruction symptoms as well as a reduction in total polyp grade, according to a release.
"Chronic nasal inflammatory diseases are an extremely common, if underappreciated, problem and there is surprisingly little late-phase research to develop new treatments. Too many patients with these chronic diseases still have symptoms, despite the availability of current medications," OptiNose President Ramy Mahmoud said in a statement. "We believe today's results--if confirmed--could be an important step forward in meeting the needs of those patients who are suffering day in and day out with fairly severe symptoms."
OptiNose's migraine treatment is currently in the hands of Avanir Pharmaceuticals, which in 2013 licensed the candidate for up to $110 million overall, which includes the use of its delivery technology. Avanir filed an NDA in the U.S. with a projected approval in the second half of this year. Other candidates for autism and sinusitis are in early and middle stages in the clinical process.
The delivery tech employs both a mouthpiece and nosepiece. The patient blows into the mouthpiece to send the treatment into the nasal cavity. By blowing out, the patient closes the pathway into the lungs so that the drug remains in the nasal cavity where it is most effective.
Additional Phase III trials are expected later this year for OPN-375.
- here's the release