 |
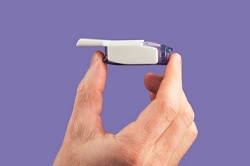
| Mannkind's Afrezza inhaled insulin drug/device combination--Courtesy of Mannkind |
Mannkind ($MNKD) endured a grueling 10-year battle for FDA approval of its fast-acting inhaled insulin drug/device combination product, Afrezza. Now the dilemma is how to sell it. The company needs to find a marketing partner fast.
Pfizer's ($PFE) infamous inhaled insulin flop Exubera casts a long shadow. It was pulled from the market in 2008, after less than a year on the market. But there are important differences between the two companies' drug delivery devices.
Exubera's inhaler was the size and shape of a Pringle's can while Afrezza's fits in the palm of your hand.
Moreover, Exubera's failure was a result of poor commercial execution, not just a poor product, said Goldman Sachs analyst Jami Rubin in a Medical Marketing & Media article. "Samples were sparse, the TV ads were late, and they were too benign and not exciting. They [Pfizer] did not court the nurses, the certified diabetic educators, who play an even bigger role than physicians in deciding to put patients on insulin. They ignored them," she said.
One drawback of the Afrezza inhaler is its potential to cause lung complications; the product carries the most severe, black box warning against use in patient's with a history of asthma and COPD. More damaging may be a warning calling for a lung test prior to taking Afrezza, and every 6 months thereafter. This could deter potential marketing partners.
Who are they? The three big diabetes players are Eli Lilly ($LLY), Novo Nordisk ($NVO) and Sanofi ($SNY), whose Apidra is behind the others in the fast-acting insulin category. Sanofi's long-acting Lantus is the best-selling diabetes drug in the world, and comes with a trove of knowledgeable sales reps. Of course, due to the silence from Mannkind, there is no way of knowing if a deal with anyone is in the making. But the company will be forced to speak by mid-August, when it releases its Q2 earnings.
In fact, CEO Alfred E. Mann told investors, "We continue to evaluate partnership opportunities and we continue in discussions with partnership partners." That was in 2005.
Earlier this year he said almost the same thing, according to The Street: "There are partners out there that recognize that this is going to be a very, very significant product. So we're not really worried. It's just a question of how we're going to launch it. There are several opportunities that we're considering."
Investors are anxiously waiting to see if Mannkind will say something new and specific in the upcoming earnings call, which must occur by August 15. They'll also consider the cash on hand (especially if there's no announcement), which stood at $35.8 million at the end of the Q1 (down from $70.8 million in Q4 2013).
- read the MM&M article
- TheStreet shows Mannkind's 10 years of marketing partnership comments
Special Report: The most influential people in biopharma today - Jami Rubin - Goldman Sachs