 |
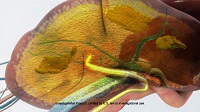
| Melphalan being delivered into the liver via a yellow infusion catheter--Courtesy of Delcath Systems |
The FDA granted Delcath Systems ($DCTH) orphan drug designation for its drug/delivery device combination product to treat liver cancer, including a rare form that affects fewer than 200,000 people in the U.S. The indication to treat intrahepatic cholangiocarcinoma will now receive favorable treatment from the FDA.
The news should offer a small degree of solace to the former high-flyer. The company's stock was once worth $180 a share, back in February 2011, before its eye drug candidate got hit with an FDA rejection. But it's now deep in penny stock territory (52 cents a share even after the good news).
Orphan drug designation qualifies Delcath for various incentives under the Orphan Drug Act, including tax credits for qualified clinical testing. But the FDA notes that "the granting of an orphan designation request does not alter the standard regulatory requirements and process for obtaining marketing approval. Safety and effectiveness of a drug must be established through adequate and well-controlled studies."
Delcath Hepatic Delivery System is for the administration of the chemotherapy drug melphalan hydrochloride against primary liver cancer, including intrahepatic cholangiocarcinoma (ICC), which is responsible for 15% of new primary liver cancer diagnoses. The company says surgical resection, or removal of cancerous tissues or tumors, is not possible in 80%-90% of ICC cases.
Delcath recently said it plans to add an 11-person cohort of patients with the ICC subtype to its global Phase II study of the candidate's effect on primary liver cancer patients. The cohort will be evaluated for tumor response rate, as measured by modified Response Evaluation Criteria in Solid Tumor, as well as progression-free survival and safety, according to the company's release.
The combination product is sold in Europe as the Delcath Hepatic Chemosat Delivery System for Melphalan, where it has had a CE mark since 2012.
Melphalan's percutaneous hepatic perfusion administration procedure involves the use of three catheters, according to the company website. First, an infusion catheter is inserted through the femoral artery and guided to the liver's hepatic artery.
Then an isolation-aspiration catheter is inserted into the femoral vein and guided into the inferior vena cava, a vein that carries deoxygenated blood from the lower body to the heart. Once in the vein, occlusion balloons are inflated to block the passage of blood from the liver to heart, isolating the liver.
Next, the infusion catheter releases melphalan directly into the liver for 30 minutes. The isolation-aspiration catheter collects the blood exiting the liver while it is passing between the occlusion balloons and send it out of the body, where it is run through Delcath's hemofiltration system to reduce the concentration of chemotherapy agents in the blood.
Finally, filtered blood is returned to the patient via a third catheter in the neck's internal jugular vein.
"We are pleased with the receipt of orphan drug designation for melphalan in the treatment of patients with cholangiocarcinoma as it is a key milestone that supports our broader regulatory and development strategy for our Melphalan/Hepatic Delivery System as a therapy for primary and metastatic liver cancers," said Delcath CEO Jennifer Simpson, in a statement. "ICC is a disease of significant unmet medical need and our Melphalan/HDS treatment may offer clinical benefit for ICC patients who face limited treatment options."
- read the release