 |
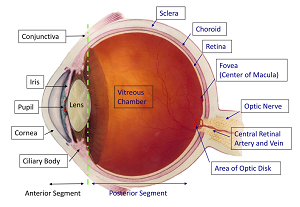
| Courtesy of National Eye Institute, NIH |
Clearside Biomedical, an Alpharetta, GA-based company focusing on back-of-the-eye delivery of drugs for macular edema, has filed for a $58 million IPO.
Clearside is fresh off positive Phase II results from earlier this month, as well as a $20 million financing. The Phase II trial found a "statistically significant mean change from baseline in central subfield thickness at eight weeks after one single treatment" in its treatment of macular edema associated with noninfectious uveitis. Uveitis is responsible for about 10% of blindness in the U.S.
The company, which got off the ground in 2011, filed with the SEC to list on the Nasdaq under the ticker CLSD.
Next on the agenda for Clearside is a Phase III trial, for which it has begun enrolling patients.
Clearside's microinjection platform administers drugs directly to the suprachoroidal space, which is between the sclera and the choroid in the eye. Targeting drugs to this space, the company says, enables access to the posterior segment ocular tissue and limits exposure, cutting down side effects in other areas of the eye.
- here's the release