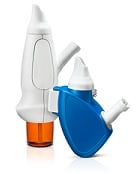
 |
| OptiNose's delivery devices with nosepiece and mouthpiece--Courtesy of OptiNose |
In 2014, the FDA rejected Avanir Pharmaceutical's intranasal candidate for the treatment of migraines, and called for additional human factors testing of the delivery device. But after a two-year delay, the FDA last week approved the med, dubbed Onzetra.
Commercial launch is expected in the coming months. It was developed by Yardley, PA's, Optinose, which then licensed the candidate to Aliso Viejo, CA-based Avanir for up to $110 million.
The meds Xsail Breath Powdered Delivery Device will administer 22 milligrams of a dry powder formulation of sumatriptan, the most commonly prescribed migraine medication.
"Onzetra Xsail provides a new and much needed treatment option for what can be a debilitating condition for millions of people," said Dr. Roger K. Cady, the director of the Headache Care Center and associate executive chairman of the National Headache Foundation, in a statement. "The Xsail Breath Powered Delivery Device allows the medication to be deposited deep into the nose, an area that is rich with blood vessels. By delivering the medication here, Onzetra Xsail provides targeted and efficient delivery with the potential for fast, consistent relief, while also limiting the amount of medicine that goes down the back of the throat."
Meanwhile, Dr. Stephen Silberstein, a principal investigator in OptiNose's clinical development program for the combination product said in a statement, "The design of the Xsail Breath Powered Delivery Device harnesses the patient's own breath to seal off the nose from the throat and deliver a low dose of a trusted medication to the richly vascular passages deep in the nose. It's an alternate approach to treating migraines that is both unique and effective."
Approval was based on safety data of more than 300 patients. In the 230-patient pivotal trial, patients were randomized to self-administer either Onzetra or a placebo. At 30 minutes, more Onzetra patients reported headache relief (41.7%) compared to the control group (26.9%). The superiority continued at every time up to 2 hours following the initial dosage, according a release.
OptiNose CEO Peter Miller said in a statement that he will use the licensing proceeds to develop its next intranasal candidate, OPN-375, designed to treat to treat patients for indications associated with chronic nasal inflammatory disease, such as nasal polyposis and chronic sinusitis.
- read the release