 |
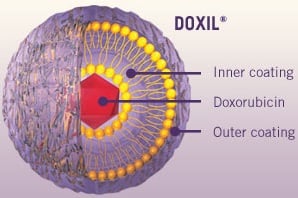
| The FDA approved a generic of Johnson & Johnson's cancer drug Doxil.--Courtesy of J&J |
It can pay to play with the FDA when it needs help with drug shortages. In a push to ease a months-long shortage of Johnson & Johnson's ($JNJ) cancer drug Doxil, the FDA fast-tracked a generic from Sun Pharma, approving it Monday.
Since February, the FDA had been allowing Sun to import its unapproved version of the Doxil substitute as one way for the agency to address the shortage.
Doxorubicin hydrochloride liposome injection sits on the FDA's drug shortage list, meaning the regulatory agency's Office of Generic Drugs can use a priority review system to bump a generic application for the drug to the front of the line. Sun's product, which will be available in 20-mg and 50-mg vials, will help patients who suffer from ovarian cancer, AIDS-related Kaposi's sarcoma and multiple myeloma.
Manufacturing problems at Ben Venue Laboratories, a unit of Boehringer Ingelheim that makes Doxil, forced the contractor to suspend operations in November 2011, exacerbating supply issues for the drug. By February 2012, FDA Commissioner Margaret Hamburg had called upon Sun Pharma to import Lipodox, a Doxil substitute that the FDA had not approved. The company manufactured the injection at an FDA-approved facility in India.
"The agency is committed to doing everything we can to address drug shortages so that patients can get the medicines they need when they need them," said Capt. Valerie Jensen, director, drug shortage staff at FDA. "For the past year, the FDA has been working to ensure that supplies of doxorubicin HCl liposome injection were not interrupted."
That work wasn't seamless, though; the FDA searched for substitutes and solutions for shortages, while a U.S. House committee criticized its regulatory aggressiveness. The committee blamed the agency for four plant closings in which production interruptions created or exacerbated shortages of many drugs. The FDA responded by saying such action was necessary to preserve patient safety.
Ben Venue announced a consent decree with the FDA regarding its Bedford, OH, plant last month. The decree bans the plant from making some drugs until the agency is satisfied that problems at the plant--more than 100 basic preventative maintenance activities hadn't been taken care of for at least a month--are cleared up. But the FDA will let the plant manufacture 100 drugs considered "essential for patient care." The contractor said it invested more than $300 million to upgrade its Ohio plant and began limited production in October.
The FDA said today that it "intends to continue exercising enforcement discretion for importation of Lipodox" until sufficient supplies of the approved generic are available.
- read the FDA release
- get more from Reuters
Special Report: Boehringer Ingelheim, Ben Venue Laboratories - Fierce's 2012 Top 10 FDA Red Flags