As the Zika virus continues to grab headlines and its spread threatens, governments and industry are urgently committing resources and analyzing vaccine development options.
On Monday, the World Health Organization said it expects the virus, which is transmitted by the Aedes aegypti mosquito, to spread across the Americas except for in Canada and Chile. So far, the United States and Brazilian governments have committed to funding research toward a vaccine. On the pharma side, GlaxoSmithKline ($GSK) is exploring using its vaccine tech on Zika, and Reuters reports that Sanofi ($SNY) is reviewing its options.
"We don't currently have any Zika vaccine candidates in development," GSK spokeswoman Anna Padula wrote in a statement to FierceVaccines. "However, recognising the health threat it poses, we are evaluating the feasibility of starting a Zika vaccine discovery programme, based on our technology platforms which we believe could be suitable for working on this target."
Padula stressed that "vaccine development is a lengthy process, typically taking 10-15 years."
Zika is suspected to cause microcephaly, in which infants are born with smaller-than-usual brains, but that link has not been definitively shown. However, WHO Director-General Margaret Chan described the circumstantial evidence to date as "suggestive and extremely worrisome," according to a Reuters report. As of last Friday, WHO figures show that Brazil has reported 3,893 microcephaly cases, a thirtyfold increase from any year since 2010.
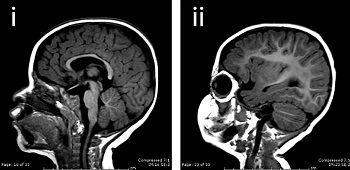 |
| A normal brain and one with microcephaly--Courtesy of Yale University |
The concerns have led scientists at the National Institute of Allergy and Infectious Diseases (NIAID) to launch a "full-court press" toward a Zika vaccine, Director Anthony Fauci told TIME. He added that since Zika's risks to infants haven't been seen in great numbers until recently, scientists hadn't been focused on the virus. Now, however, the NIAID is looking at ways to fund Zika work through grants and is examining how its research on similar viruses can be translated. Fauci cautioned, though, that the work is unlikely to impact the current outbreak in any big way.
Brazil's Butantan Institute, for its part, is embarking to develop a vaccine "in record time," though its director said the work would probably take between three and five years, according to an AP report.
Inovio Pharmaceuticals ($INO) has also announced involvement in Zika, saying on Friday it'll work with South Korea's GeneOne Life Science to develop a vaccine.
Zika's sudden spread and the tumult surrounding it have left health officials yet again stating that a change is needed in the vaccine development process. Similar to the Ebola epidemic in West Africa, there are no drugs or vaccines at the time of the outbreak, leading University of Oxford global health professor Trudie Lang to call it a "case of deja vu," Reuters reported. Because large drugmakers in some cases don't know the market implications of research into new diseases, officials have recently called for a $2 billion vaccine development fund to pay for upfront R&D costs.
- here's the Reuters story
- get more on GSK and Sanofi
- here's the TIME piece on the NIAID work
- read the AP story on Brazil's commitment
- here are Inovio's release and Glaxo's statement