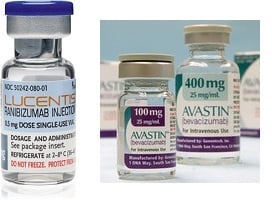
 Genentech's Avastin cost about $50 a dose when used off-label for the eye. Then came the company's similar drug Lucentis, reformulated specifically for eye treatments--and it cost $2,000 per dose. So how did the company persuade physicians to make the switch? According to The New York Times, consultation payments may have had something to do with it.
Genentech's Avastin cost about $50 a dose when used off-label for the eye. Then came the company's similar drug Lucentis, reformulated specifically for eye treatments--and it cost $2,000 per dose. So how did the company persuade physicians to make the switch? According to The New York Times, consultation payments may have had something to do with it.
Several of the top Lucentis billers are also among Genentech's highest-paid consultants, the paper notes, citing a federal database. Half the 20 docs who pocketed the most from the company to promote Lucentis were among the drug's highest 2012 users, billing for more of the drug than 75% of their fellow prescribers. And those 20 took home $8,500 to $37,000 over 5 months last year.
While many docs maintain their behavior isn't influenced by pharma company speaking and consultation fees, the payments have come into the spotlight recently with the launch of the Physician Payment Sunshine Act database. And many experts say the fees do have sway, prompting GlaxoSmithKline ($GSK)--trying to clean up its marketing act after an off-label marketing settlement and $489 million doctor-bribery scandal in China--to do away with the practice altogether.
 |
| Adriane Fugh-Berman--Courtesy of Georgetown University Medical Center |
Dr. Adriane Fugh-Berman, director of a project that educates doctors about drug marketing claims, says even gifts as small as a sandwich can influence a prescriber's psychology. "No one wants to feel like they were being bought off for $5," she told the Times. "That's why they convince themselves that the drug is better."
When it comes to Avastin vs. Lucentis, multiple studies--including a 1,200 patient, government-sponsored clinical trial that wrapped in 2011--have shown that the two meds are nearly equivalent. And while the company has touted Lucentis as a safer option--Avastin must be repackaged for use in the eye, opening up the possibility for contamination--a recent review showed that in 3,665 patients, Avastin didn't increase the risk of death or serious side effects when compared with its pricier sister drug.
Of course, plenty of doctors still use Avastin as their go-to, including those who take payments from Genentech. One Illinois-based retina specialist, who took home about $8,500 from Genentech between last August and December, told the NYT he starts patients off with Avastin and switches them to Lucentis or Regeneron's ($REGN) Eylea if they don't do well. About half of wet age-related macular degeneration patients receive the drug, with Lucentis and hard-charging Eylea sharing the rest of the market, the newspaper notes.
A spokesman for Genentech, which has also paid rebates to doctors who use large amounts of Lucentis in order to win scripts for the $1.3-billion-a-year seller, told the Times that the company chooses its doctor-speakers based on their clinical know-how. "We're looking to work with doctors who are in the top of their field, and who can provide us an honest perspective on what patients need and don't need," he said. A company spokeswoman told FiercePharma Genentech fully supports efforts to increase transparency around the collaboration between the industry and healthcare professionals, and it doesn't believe physicians make treatment decisions based on these relationships.
Abroad, Genentech parent company Roche ($RHHBY) has run into allegations that it and marketing partner Novartis ($NVS) colluded to promote the pricey treatment over Avastin. Last week, an Italian regional court upheld charges levied against the pharma giants earlier this year, fining the pair €180 million ($221.6 million) for manipulating sales. Authorities in France and the EU are also currently looking into the matter.
- read the NYT story (sub. req.)
Special Report: Top 10 pharma companies by 2013 revenue - Novartis - Roche