Sometimes it seems that the FDA has a scattershot approach to policing pharma marketing, with warning letters few and far between--and some of them lobbed from left field. But there's one tried-and-true way to invite unwanted attention from the agency's marketing police: Leave side effects out of drug promos, according to an analysis presented at a recent conference.
A team of execs at Johnson & Johnson ($JNJ) looked at FDA warning letters and untitled letters issued by the agency's Office of Prescription Drug Promotion (OPDP) from 2013 to 2015, and found that 60% of the letters cited pharma for omitting risk information in promotional materials, Medical Marketing & Media reports.
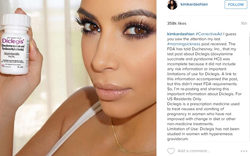 |
| Kim Kardashian issued a corrected social media post for Diclegis with the hashtag "CorrectiveAd" on Instagram. |
We have one high-profile--and recent--example, thanks to the Canadian pharma Duchesnay's marketing of its morning sickness med, Diclegis. The company signed on celebutante Kim Kardashian to promote its med over the summer, with the cover girl taking to Instagram and other social media outlets to get the word out.
But the FDA quickly picked up on one key fact: Kardashian's now-infamous Instagram post didn't mention Diclegis' risks or limitations. In August, the agency chastised Duchesnay in a warning letter, demanding that it run corrective ads. Kardashian subsequently issued corrective messages on Instagram, Twitter and Facebook.
But while Duchesnay is the latest drugmaker to feel the agency's burn, 8 other companies received a warning letter or untitled letter from OPDP this year, including Actavis and Japanese pharma Otsuka. Last year, the OPDP called out 10 companies for marketing offenses--a relatively low number, considering that the FDA's promotional watchdogs issued 19 letters or violation notices in 2007.
When it comes to slapping down marketing infractions, the agency may be more likely to resort to a warning letter if the product has a black-box warning. In 2013, FleishmanHillard's Mark Senak looked at a database of warning letters and untitled letters from 2004-2013, and saw that drugs with black boxes were more often singled out for the more serious warning letter. Among the citations issued over black-box drugs, 45% were warning letters, while warning letters accounted for only 32% of the citations over drugs without them. Senak published his findings at the Eye on FDA blog.
The J&J analysis also comes as the FDA refines its position on social media marketing. The agency issued 19 warning or untitled letters for digital, social media or mobile promotional activities between 2013 and 2015 compared to 25 for print materials, the MM&M article points out, as more companies shift to digital advertising.
Last year, the agency unveiled new social media guidance that would limit drugmakers' marketing through outlets such as Facebook and Twitter, provoking fighting words from industry groups that called the measures too restrictive. And some lawmakers are pushing back, with a Missouri congressman introducing a bill earlier this year that would allow drugmakers to use hyperlinks for risks and benefits in online promos rather than spelling them all out in posts.
- here's the MM&M article (reg. req.)
- get the FDA's list of warning and untitled letters
- read the Eye on FDA blog post
Special Report: The top 10 pharma companies in social media