 |
| Courtesy of BioDelivery Sciences |
Squeezing it in at the end of the year, Endo Pharmaceuticals ($ENDP) and BioDelivery Sciences ($BDSI) filed an NDA with the FDA for the companies' buccal Buprenorphine painkiller.
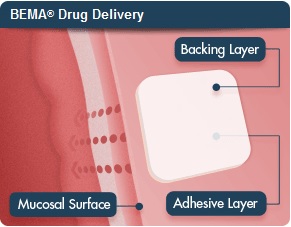
More than two years ago, BioDelivery and Endo partnered with a $180 million deal for the use of BioDelivery's BioErodible MucoAdhesive (BEMA) technology to deliver drugs across mucosal surfaces like the inside of the cheek. The bioerodible film is placed on the inner lining of the cheek and quickly delivers a dose of the drug buprenorphine across the mucous membrane. According to the company, the process takes about 15 to 30 minutes.
Endo had failed to get the drug past a critical Phase III study prior to the partnership. But with Raleigh, NC-based BioDelivery's technology in tow, Endo's Phase III hit its primary endpoint when compared to a placebo. That triggered a $10 million milestone payment for BioDelivery Sciences and a surge in its stock price, not to mention a renewal of faith in the BEMA platform.
"The submission of the NDA for Buprenorphine HCl Buccal Film is a major milestone in BDSI's partnership with Endo Pharmaceuticals," BioDelivery CEO Mark Sirgo said in a statement. "Buprenorphine's role in pain management in the United States has always been hampered by the lack of a convenient and flexible dosage form. We believe our patented BEMA technology will overcome this obstacle and provide physicians with a meaningful product to treat chronic pain. We look forward to continuing our work with Endo, and making this therapy available to patients who need it."
Ireland's Endo has suffered at the hands of pain generics recently, but the candidate has garnered sales estimates of about $300 million contingent upon the FDA approving the just-submitted NDA. The potential windfall, though, could be dampened by new products from Teva ($TEVA) and Pfizer ($PFE) that could soon enter the pain market.
- here's the release