 |
|
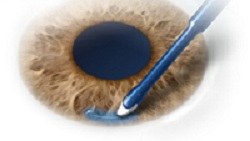
ReSure Sealant is applied directly to the cornea.--Courtesy of Ocular Therapeutix |
Ocular Therapeutix is formally seeking premarket approval with the FDA for its hydrogel-based sealant used to protect the eye after cataract surgery.
Ocular's ReSure Sealant is designed to deliver a hydrating gel material that prevents wound leaks by creating a temporary covering over surgical incisions in the cornea after an operation. The company's polymer-based, sustained-release hydrogel technology, ReSure, dissolves over time and exits the eye via the patient's tears.
Ocular hopes to launch ReSure in the clinic later this year if the FDA approves it. It would be the first and only sealant to gain such approval for ophthalmic use.
The FDA will look at the results of a 488-patient pivotal trial, in which researchers compared ReSure to sutures for 7 days following cataract surgery. The desired end result is to cut down on wound leaks, which are widely believed to be the culprit behind various post-operative complications, according to the company.
Other eye drug-delivery innovations Ocular has in its pipeline include plugs inserted into the eye's punctum for the treatment of glaucoma, ocular hypertension and bacterial conjunctivitis, as well as a hydrogel-based intravitreal injection for the sustained delivery of ophthalmic medications.
- here's the release