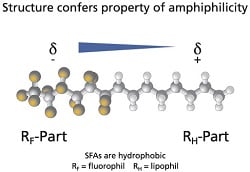
 |
| Semifluorinated alkanes (SFAs) provide a platform for insoluble drugs.--Courtesy of Novaliq |
Eye drug delivery outfit Novaliq touted a positive Phase I for its cyclosporin eye drops designed to treat dry eye disease. Novaliq's semifluorinated alkane technology allows for a clear solution of cyclosporin, which is poorly soluble in water, thus making for a more effective eye drop that doesn't impair vision, the company says.
In the early-stage study of its CyclASol eye drops, the 18 patients involved showed no drug-related symptoms, according to a release, and no reported discomfort, including "dryness, grittiness, burning, stinging, tiredness, blurred or foggy vision, redness, watery eyes, eye mucus or crusting." These are some of the side effects often associated with cyclosporin when administered as an emulsion as opposed to a dissolved solution made possible with the semifluorinated alkanes.
"For patients with dry eye disease, there are few approved drug options available," Chief Scientific Officer Dieter Scherer said in a statement. "Cyclosporin is a well-accepted active drug substance for this disease, although current formulations come with several limitations including side effects and limited patient acceptance due to cyclosporin's poor solubility."
Novaliq brought in an $18 million round last year from entrepreneur Dietmar Hopp's Dievini Hopp BioTech Holding, which also contributed the rest of a total $35 million in financing. Most of that latest round went into spurring CyclASol into its clinical trial.
Also making use of the alkane platform is Novaliq's over-the-counter NovaTears, which secured a CE mark last summer.
- here's the release