 |
| Courtesy of NanoSmart |
California's NanoSmart netted an orphan designation to treat the rare Ewing's sarcoma, a childhood bone cancer, with a product that makes use of the company's targeted liposomal nanoparticle delivery platform. In what is NanoSmart's second such designation from the FDA, the company is qualified once again for certain incentives, including 7 years of market exclusivity and a subsidized trial process.
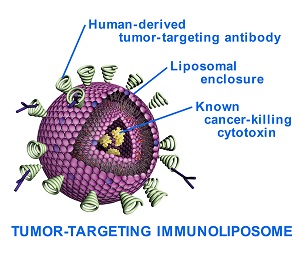
NanoSmart's formulation is led by the cytotoxic agent dactinomycin, which is known to be effective in patients with Ewing's sarcoma, but because it comes with toxicity issues and lifelong complications, it is often used sparingly and as a late resort. But encased in NanoSmart's liposomal vehicles, the powerful drug can be targeted to the tumor site specifically, reducing side effects and potentially allowing for higher doses.
The company's main focus is its human-derived anti-nuclear antibody, which targets areas of necrosis in many types of tumors, according to its website. And putting this antibody on the surface of a drug-delivery particle gives it the mechanism needed to anchor at the tumor site and deliver its payload more efficiently.
The FDA earlier this year gave NanoSmart an orphan designation for its similarly delivered formulation of doxorubicin, another common cancer killer. The treatment targets Ewing's sarcoma as well, with possible indications elsewhere, the company said at the time.
"The FDA's commitment to supporting drug development for rare and pediatric diseases has created opportunities for our industry to efficiently develop new therapies for patients that are most in need," NanoSmart President James Smith said in a statement. We are very pleased that the FDA has issued this designation and we look forward to commercializing these exciting drug delivery platforms."
The administration's orphan designation is reserved for treatments of diseases that affect fewer than 200,000 patients in the United States, with an eye toward making these targets more economically feasible for companies to pursue for the amount of time it takes to develop such products.
NanoSmart has teamed up with the likes of UCLA and the Children's Hospital of Los Angeles in the last 5 years as it worked to develop its proprietary platform.
- here's the release