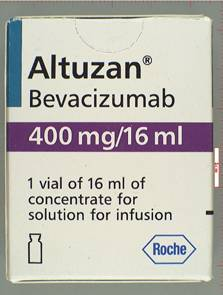
 Like a whack-a-mole puppet, fake Avastin has surfaced again. This time, the counterfeit version of Roche's ($RHHBY) cancer drug came from a different distribution network, and it was packaged differently, in boxes labeled with Altuzan, the drug's brand name in Turkey. Two things were the same: The pseudo-Avastin product contained none of the real drug's active ingredient, and medical practices in the U.S. bought it.
Like a whack-a-mole puppet, fake Avastin has surfaced again. This time, the counterfeit version of Roche's ($RHHBY) cancer drug came from a different distribution network, and it was packaged differently, in boxes labeled with Altuzan, the drug's brand name in Turkey. Two things were the same: The pseudo-Avastin product contained none of the real drug's active ingredient, and medical practices in the U.S. bought it.
According to the FDA, this latest incarnation came from an overseas supplier, Richards Pharma, which also operates under the names Warwick Healthcare Solutions, Ban Dune Marketing, and Richards Services.
The Wall Street Journal tracked Ban Dune to La Jolla, CA, but the company shut down in August. In January, a St. Louis doctor and two Ban Dune staffers were indicted in the U.S. on charges of selling adulterated cancer drugs, including versions of Roche's Herceptin and Rituxan, and Amgen's ($AMGN) Neupogen. The two Ban Dune employees have pleaded guilty. The drugs Ban Dune distributed also had Turkish labels.
The latest batch of phony Avastin again points out the vulnerabilities in pharma's global supply chain, Reuters notes. And this is true, of course; tracking and oversight could use a boost. But the counterfeit drug trade is a two-way street. As the WSJ reports, prosecutors called Ban Dune's pricing "too good to be true." In the previous counterfeit-Avastin case, the cost for the fake was several hundred dollars per vial less than the real thing's sticker price. Regulators and pharma security teams can hunt down the suppliers, but if doctors weren't willing to buy from questionable suppliers, the hunt might not be necessary. (Image courtesy of the FDA.)
- read the WSJ piece
- get more from Reuters