InSite Vision, working off its eye drop platform with polymer-suspended drug delivery, is planning to submit an NDA in 2015 for its treatment for blepharitis, an eyelid inflammation disease for which there is currently no FDA-approved treatment.
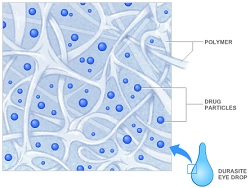 |
| DuraSite platform with suspended drug particles for consistent delivery--Courtesy of InSite Vision |
InSite's DexaSite delivers the drug dexamethasone embedded in the company's polymer matrix eye drop platform DuraSite. The drug particles are suspended evenly in a network of cross-linked polymers, allowing it to be released slowly over time. The company touts four to 6 hours of consistent delivery. Past safety studies have shown the platform to be nontoxic and biocompatible, the company says.
In a Phase III study for blepharitis, DexaSite showed successful superiority versus vehicle data over 15 days with twice-daily dosing. In the study, patients reported a reduction in irritation symptoms of the disease. The company said it believes it's the first significant Phase III symptom improvement for blepharitis.
The FDA told InSite that the full results of the late-stage study will be enough for marketing approval. The company will also be aiming for European regulatory action later this year.
"Blepharitis is estimated to affect 34 million patients in the U.S., and there is currently no approved therapy indicated to address this chronic inflammatory eye condition," InSite Chief Medical Officer Dr. Kamran Hosseini said in a statement.
- here's the release