 |
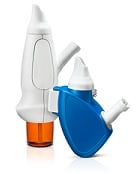
| Avanir's and OptiNose's powder delivery devices with nosepiece and mouthpiece--Courtesy of OptiNose |
California drugmaker Avanir Pharmaceuticals ($AVNR) touted the FDA's acceptance of its application for an inhaled migraine treatment after a pivotal trial.
The inhaled, dry-powder sumatriptan treatment, which makes use of Avanir partner OptiNose's bidirectional nasal technology, is now set for a review date in November this year.
In July of last year, Avanir offered PA-based OptiNose $20 million up front with another $90 million in milestone and sales target payments on the line. In late 2012, the drug-delivery device demonstrated relief of headaches in 68% of patients as quickly as 15 minutes after treatment. If approved, AVP-825 would be the first sumatriptan migraine powder on the market.
The device itself uses a patient's natural breathing to propel drugs into the nasal cavity. A patient blows into the mouthpiece to send drugs through a nosepiece. With this bidirectional technology, the soft palate closes, which prevents the unwanted propulsion of active ingredients into the lungs, according to Avanir.
Back in July, Optinose CEO Peter Miller said in a statement: "Avanir is an ideal partner given its proven track record of successfully developing and commercializing neuroscience products. The results from our Phase III clinical study were extremely encouraging and we believe we have a potential treatment that provides pain relief quickly and with few adverse events."
- here's the release