 A new batch of counterfeit Avastin found in the U.S. has been tied to a company that marketed unapproved drugs from unapproved foreign manufacturers and shipped them by methods that violate just about every rule of safe product handling.
A new batch of counterfeit Avastin found in the U.S. has been tied to a company that marketed unapproved drugs from unapproved foreign manufacturers and shipped them by methods that violate just about every rule of safe product handling.
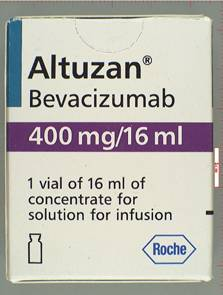
The FDA reported late Tuesday that it had recovered boxes labeled Altuzan, which is the the brand name for Avastin in Turkey. Tests, however, determined there was no active ingredient in the counterfeits, The Wall Street Journal reports. There have been no reports of problems related to the drugs, according to the FDA and Genentech, the Roche ($RHHBY) unit that makes the cancer treatment.
Like the earlier batch discovered in February, the fake was being marketed to medical practices, presumably at deep discounts. The FDA warns any practices that have obtained drugs "through foreign sources, in particular from Richards Pharma, also known as Richards Services, Warwick Healthcare Solutions, or Ban Dune Marketing Inc (BDMI)" that they should alert the agency's criminal investigation unit.
The WSJ points out that two Californians associated with Ban Dune pleaded guilty to federal charges in February for selling unapproved drugs to a physician in the St. Louis area. James Newcomb admitted in court to selling "adulterated" versions of cancer drugs, including Neupogen, Herceptin and Rituxan. The St. Louis Post-Dispatch reports that one shipment "arrived with a ruptured cold pack that turned it into a gooey mess."
While the case does not mention Altuzan, it says Ban Dune shipped other drugs with Turkish labels from unapproved manufacturers.
Concern over mishandled drugs of any kind, fake or stolen, was expressed by the FDA late last month when it issued a new standard operating procedure in the case of pharmaceutical cargo theft. The new SOP lays out new procedures for manufacturers, including notifying the public of the potential health risks from mishandled drugs that might make it back into the distribution chain. The FDA even says that if manufacturers are not forthright enough about the thefts, the FDA would take upon itself to alert the public.
The problem is that more fakes, especially of life-saving medications like cancer treatments, are proliferating, and this fact is raising questions among regulators, legislators and manufacturers about what else can be done to prevent some medical tragedy. (Image courtesy of the FDA)
- here's the FDA release
- read the WSJ piece
- get the The St. Louis Post-Dispatch story