 |
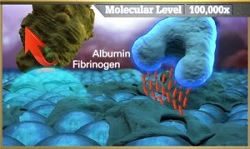
| CeloNova's Polyzene surface technology binds to albumin and resists platelets--Courtesy of CeloNova |
CeloNova BioSciences won U.S. approval to begin an investigational device exemption trial for its coronary stent system designed to improve stents using a nano-coating particle technology.
The San Antonio, TX, company's Cobra PzF system, already CE marked, is a cobalt chromium alloy with a nano-thin coating of the company's Polyzene polymer technology, which the company says both prevents the formation of blood clots at the site of the stent and keeps the blood vessel from narrowing again during and after the procedure.
The Polyzene technology, according to the company, coats the Cobra stent and its tiny particles bind to albumin in the blood. At the same time, the surface resists platelets and fibrinogen, preventing them from accumulating at the site. According to the company's website, the Polyzene "virtually eliminate(s) the inflammatory response."
 |
| CTO Jane Ren--Courtesy of CeloNova |
"The Polyzene-F nano-coating technology, when combined with a next-generation stent and delivery system, is a potential game-changer," CeloNova Chief Technology Officer Jane Ren said in a statement. "Early indications are that the nano-coated stent leads to a much faster, natural healing of the artery and also would reduce the need for long-term blood-thinning medication."
The company is also performing additional studies along with the IDE trial.
Just last week, CeloNova won the FDA's 510(k) clearance for its Embozene microspheres, which are designed, in part, for the treatment of uterine fibroids.
- here's the release