 BioDelivery Sciences' ($BDSI) Onsolis, a cancer pain treatment that makes use of the company's buccal delivery platform, picked up a new FDA approval for a reformulation of the treatment, which is expected to return to the U.S. market in 2016. BioDelivery earlier this year pulled out of a commercialization partnership with Sweden's Meda, which decided to focus more on respiration, and BioDelivery is currently looking for a new partner.
BioDelivery Sciences' ($BDSI) Onsolis, a cancer pain treatment that makes use of the company's buccal delivery platform, picked up a new FDA approval for a reformulation of the treatment, which is expected to return to the U.S. market in 2016. BioDelivery earlier this year pulled out of a commercialization partnership with Sweden's Meda, which decided to focus more on respiration, and BioDelivery is currently looking for a new partner.
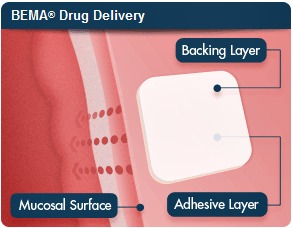
The FDA originally approved Onsolis, a fentanyl buccal soluble film for the treatment of breakthrough pain in patients with cancer, in 2009. BioDelivery's BioErodible MucoAdhesive (BEMA) technology delivers drugs to the mucosal membrane on the inner lining of the cheek, giving the treatment direct access to the bloodstream with a simple delivery mechanism.
The new formulation of Onsolis addresses some concerns from the FDA about the coloring of the strip, which is pink on one side and white on the other. Because of fading, some patients had trouble discerning which side contained the active ingredient, and BDSI said they have fixed the strips to be more intuitive.
"We are pleased to have obtained FDA approval of our sNDA and to now be in a position to move toward returning ONSOLIS to the U.S. marketplace," CEO Mark Sirgo said in a statement. "ONSOLIS remains an important differentiated fentanyl containing product for this indication given that it is the only product for buccal administration, providing patients with an alternative dosing option."
Of the prospect of a new partnership, Sirgo continued: "Although we have options for ONSOLIS, including commercializing it on our own, our current plan is to determine the value we can secure in a partnership with a company that has access to the target physician audience. We have been engaged with a number of potential partners, and with this approval, we can now proceed forward with those discussions in earnest. We will provide more definitive timing in the near future about the reintroduction but this would not be prior to 2016."
- here's the release