 |
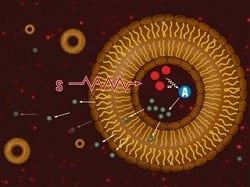
| A liposome releases chemo when hit with an X-ray.--Courtesy of Boise State |
Combining chemotherapy and radiotherapy would be ideal for cancer treatment, but administering both at the same time can lead to unacceptable and often deadly levels of toxicity. Now researchers have developed liposomes that contain a chemotherapy drug activated by radiotherapy, offering both cancer-killing power and a targeted approach in a more controlled manner.
In initial studies using FDA-approved drugs, the team from Boise State and the University of Arkansas designed a simple bilayered liposome with nanoparticles inside its cavity. When these nanoparticles are hit with X-rays, they emit ultraviolet light, which then triggers the release of phospholipase A2, a fat-eating enzyme, from additional particles in the vehicle. Because the liposome's walls are themselves made up of fat, the enzyme breaks through the shell and allows chemotherapy particles, also contained within the liposome, out into the open.
Thus, the liposome can be safely nestled in a tumor before it is activated by the radiation, keeping healthy cells safe from the deadly drugs and tumors fully bathed in it. Daniel Fologea of Boise State and co-authors from Arkansas won a patent for the cancer-killing liposome in August.
"The liposomes are designed to release their precious cargo upon exposure to X-ray," Fologea said in a statement. "Not only does this target where the medication goes, it also allows for a huge concentration of the drug to be released at once at the tumor site, thus increasing its efficacy. In addition, this combined modality of treatment employing concomitant radio and chemotherapy is supra-additive, which means it is several times more efficient than each therapy applied alone."
Though in its very early stages, the researchers are looking to improve its mechanism of action to eventually get the technology to future clinical testing. Adding antibodies to the surface that would allow the delivery vehicles to recognize cancer cells in the body would make them even more highly targeted. Once they reach their target, a burst of X-rays would finish the job.
- here's the Boise State article