 |
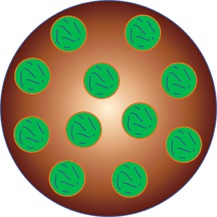
| A diagram of a microparticle (brown) holding nanoparticles (green), which hold a dose of drugs to be released over time.--Courtesy of Jordan Green, Johns Hopkins |
A needle in the eye is a notoriously unpleasant experience, albeit a necessary one for the treatment of macular degeneration, a condition that can lead to vision loss. And now researchers at Johns Hopkins University have developed a time-release nanoparticle coating to hold drugs in the back of the eye longer, cutting down on the number of injections a patient will need.
Neovascular, or "wet," age-related macular degeneration causes vision loss as blood vessels grow at the back of the eye. Protein-based drugs have been found to adequately treat this blood vessel growth, or angiogenesis, according to a study to be published in the October issue of the journal Biomaterials, but patients require injections as often as once a month for the drug to remain effective.
To overcome this barrier, the Johns Hopkins team developed a short-stranded protein that blocks the growth of unwanted blood vessels, as reported by the university. The scientists found in animal tests that the drug was as effective as the current standard of care but that the eye flushed it out in about a month.
So they used two different packing mechanisms to protect the drug for longer periods of time. First, they encapsulated the drug itself in a biodegradable nanoparticle designed to break down in the eye's environment over time, releasing the drug in small amounts. Then, to enhance the time-release delivery even further, they packed those nanoparticles into larger microparticles, also with the ability to break down over time.
With this delivery method, the drug lasted for at least 14 weeks at a time in animal models, the university reports.
"We hope that our system will work in people, and make invasive treatments much less frequent, and therefore easier to comply with, and safer," said lead author Jordan Green in a statement.
- here's the Johns Hopkins report
- and here's the Biomaterials abstract