 |
| University of Delaware's Thomas Epps |
As drug delivery becomes more and more dependent on nanotechnology for treatments such as chemotherapy, it's important that scientists in the field understand the implications of the tiny vehicles and what factors might play a part, for good or bad, in their delivery potential.
In a paper published in Nature Communications, researchers from the University of Delaware showed that routine procedures can change the size and shape of nanoparticles, which can then affect how those nanoparticles deliver their payload.
Using techniques such as cryogenic transmission electron microscopy, small angle X-ray scattering, small angle neutron scattering and dynamic light scattering, the researchers mimicked preparation conditions often used in the lab to make the nanoparticles, according to a report from the university.
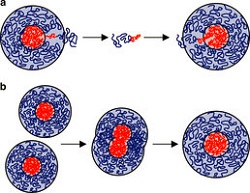 |
| Two common mechanisms by which nanoparticles undergo growth when agitated--Courtesy of University of Delaware |
The growth of the nanoparticles, called micelles, commonly occurs when either one part of a micelle breaks off and attaches to another or when two micelles fuse together to make one larger one. In both cases, the uniformity is compromised.
"At 75 nanometers, a nanocarrier may deliver its cargo directly to a tumor," lead researcher Thomas Epps said in a statement. "But with vigorous shaking, it can grow to 150 nanometers and may accumulate in the liver or spleen. So simple agitation can completely alter the distribution profile of the nanocarrier-drug complex in the body."
This research could help develop safer methods of manufacturing nanoparticles so they can be of a uniform size, which is optimal for their delivery function. Before, Epps says, engineers were working under the assumption that the vehicles were stable upon entering a solution when, in fact, they are still vulnerable to agitation.
- here's the University of Delaware report
- and here's the abstract