 |
| Polymeric micelle delivery platform--Courtesy of UW-Madison |
University of Wisconsin-Madison spinout Co-D is deploying its polymeric micelle drug delivery platform with the aim of commercializing a failed Bristol-Myers Squibb ($BMY) cancer drug. So-called Triolimus boasts FDA's orphan drug designation to treat the rare blood vessel cancer angiosarcoma, a milestone that will accelerate its path to FDA approval (or rejection).
As implied by the name, Triolimus consists of three drugs: paclitaxel, rapamycin (also known as the generic sirolimus) and tanespimycin. BMS halted development of tanespimycin alone in 2010 while it was in Phase III trials for the treatment of multiple myeloma because the drug was nearing its patent expiry and was difficult to manufacture. In spite of some promising trial data, all work on tanespimycin was brought to halt--not just that on the Phase III trial.
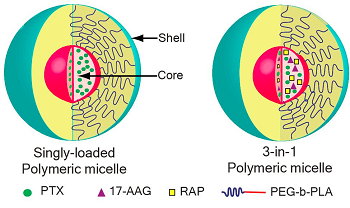
Co-D believes it can the overcome the formulation challenges of tanespimycin and delivery challenges of rapamycin using, you guessed it, drug delivery. Micelles are lipids that arrange themselves in a spherical form in aqueous solutions; they consist of a hydrophilic (water-loving) exterior and a hydrophobic (water-hating) interior, making them a good delivery vehicle for water insoluble drugs.
Co-D says that its polymeric micelles can package multiple complimentary drugs to deliver a synergistic punch to cancer. "Moreover, combined delivery may allow a single rapid infusion to substitute for the sequential slow infusions often required in traditional combined chemotherapy regimens," the company website says.
The platform apparently has interesting personalized medicine applications as well.
"If a particular patient's tumor is known to be sensitive to two conventional agents in combination at high dose and one conventional agent at low dose, a three-drug polymeric micelle formulation can be designed to deliver the optimal ratio of individual drugs for a particular patient," the website says.
MedCity News reports that Co-D just raised $145,000 in debt as it ramps up its preclinical efforts. Orphan drug designation for treating angiosarcoma was granted in April 2015. The candidate's applications to treat breast cancer and non-small cell lung cancer are also being studied preclinically.
Generic paclitaxel is one of the most commonly used cancer drugs, while Pfizer's ($PFE) rapamycin drug Rapamune last year became the first med approved to treat lymphangioleiomyomatosis, a rare, progressive lung disease that primarily affects women of childbearing age, according to the FDA.
Meanwhile, the list of trials of tanespimycin is long, but no one, including BMS, has been able to commercialize the drug.
- here's MedCity News' take