As the global alarm continues to sound about the Zika virus, Sanofi ($SNY) said on Tuesday that it's getting involved. In doing so, it became the first major pharmaceutical company to commit to Zika vaccine R&D, launching a program to develop a jab just after receiving the first regulatory approvals for its dengue shot.
 |
| Sanofi Pasteur's Nicholas Jackson |
The French pharma said it'll seek to apply its research and infrastructure developed in its 20-year dengue vaccine pursuit to Zika, which is spread by the same species of mosquito. And, in speaking to the Wall Street Journal, Nicholas Jackson, Sanofi Pasteur's global head of research--who will lead the program--said "Theoretically, there could be some cross immunity."
So far, Sanofi is the largest company involved in Zika vaccine R&D, although GlaxoSmithKline ($GSK) said it is evaluating its technology for a Zika jab. Biotechs such as Inovio Pharmaceuticals ($INO), NewLink Genetics ($NLNK) and Hawaii Biotech have also gotten involved.
Sanofi's move comes one day after the WHO declared the virus an international public health emergency due to its recent suspected link to microcephaly, allowing the agency to mobilize resources against it. The last such declaration was during the Ebola outbreak.
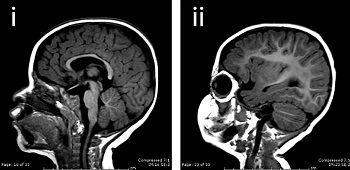 |
| A normal brain and one with microcephaly--Courtesy of Yale University |
Zika hasn't been a priority for vaccine developers until recently because it usually causes mild fevers and rashes; 80% of those affected don't even show symptoms, Reuters reports. But a new flare-up of microcephaly cases in Brazil that is suspected to be linked to the virus has elevated the alarm.
To date, no effective drugs, diagnostics or vaccines for Zika exist. A vaccine could take 10 to 15 years to develop, though some companies and organizations have said they may be available quicker. Inovio has said it may be able to test its vaccine later this year, while Brazil's Butantan Institute said it intends to develop its vaccine in 3 to 5 years.
Zika's rapid spread could provide biopharma companies with a blockbuster opportunity, the WSJ reported, depending on the timing of a potential launch and support by governmental vaccination campaigns.
- here's the Reuters story
- get more from WSJ (sub. req.)